Carbon
| Carbon | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::12.0107 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Nonmetals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Black (graphite--shown below) Clear (diamond) 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 14, 2, p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | 1s2, 2s2, 2p2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 4 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-44-0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density | Density::0.002267 g/ml | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::3823 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::4300 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Carbon | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Carbon is one of the most important elements in chemistry and is often called the basis of life because all living organisms contain carbon. This amazing element can be found all around us: in the air we breathe, the foods we eat, and even in our DNA. Its versatility allows it to form literally millions of chemical compounds, many of which are essential for life. In fact, carbon has such a vital role in chemistry that it has its own branch of study--organic chemistry.
Properties
Carbon is found in group 14 and period 2 on the periodic table. A carbon atom has six electrons, two in the inner shell and four in the outer shell. Having four valence electrons allows carbon to form a wide variety of compounds, and it can either gain or lose electrons when bonding, depending on the electronegativity of the other element(s). It is a nonmetal and a solid. Interestingly, when carbon is heated at normal pressures it undergoes sublimation instead of melting; that is, it changes directly from a solid to a gas. However, it can be melted, and the melting point given in the table is for carbon heated at a pressure of less than 10 atmospheres[1].
Carbon, in its many different forms, can have a wide range of properties. For example, graphite is very soft and slippery (0.5 mohs on the hardness scale) while diamond is one of the hardest known substances (10.0 mohs). Most forms of carbon are nontoxic (although inhaling too much soot/carbon dust will irritate the lungs), but carbon monoxide (CO) and cyanide (CN) are poisonous and deadly. Graphite is black and diamonds are transparent. Some isotopes of carbon are essential for life, while others are radioactive. Diamond has a higher thermal conductivity than any other element, but nanofoam is a great insulator[1]. Thus, each allotrope and compound of carbon has its own unique properties based on the arrangement of the carbon atoms.
In addition to different allotropes, carbon has several isotopes. A normal carbon atom, carbon-12, has six protons and six neutrons. This is the most common isotope, making up about 98.9% of all carbon atoms[2]. The carbon-13 isotope has seven neutrons, and carbon-14 has eight neutrons. Carbon-12 has been used as the basis for atomic weights by IUPAC (International Union of Pure and Applied Chemistry) since 1961. Carbon-14 has a 5730-year half-life and is used in carbon dating[3]. Other isotopes of carbon are radioactive with very short half-lives. Carbon-11 (five protons) has a half-life of twenty minutes. It is used primarily in the medical field for finding tumors. In positron emission topography (PET), a body scanner detects gamma rays given off as the carbon-11 decays and highlights the area in the body where the tumor is[4]. Other isotopes of carbon have half-lives that are only seconds and even milliseconds long (see the table to the right for a more complete list)[5][6].
History
Man has long been familiar with various forms of carbon, such as soot, charcoal, graphite, and diamond. In ancient times, carbon black was used as ink for writing, and natural gas was used for lamp oil as far back as the fourth century B.C.[7] Charcoal was used for medicinal purposes starting in 1500 B.C. to treat putrefying wounds and a number of other ailments, including epilepsy, chlorosis, and anthrax[8]. The name "carbon" comes from the Latin word carbo, meaning charcoal. The names for graphite and diamond come from the Greek words graphein (to write) and adamas (invincible).
It was Antoine Lavoisier, known as the father of modern chemistry, who first theorized that diamonds were composed of carbon. He, along with scientists Scheele and Berthollet, repeated an experiment first performed in 1694[4]. They bought a diamond and put it inside a glass container. They then used a magnifying glass to focus sunlight on the diamond, causing it to disappear. Lavoisier deduced that the diamond must have disintegrated into a gas that he could not see[9]. In 1796, chemist Smithson Tennet again repeated the experiment and proved that diamonds are made of pure carbon and the gas produced by burning the diamond was carbon gas that bonded with the oxygen in the glass case to form carbon dioxide. Diamonds were first synthesized in 1953 by ASEA in Switzerland. The General Electric Company also succeeded in making diamonds two years later, in 1955[4].
Thomas Edison made the first carbon fibers in 1879. He used bamboo and cotton and, by exposing them to high temperatures in a controlled atmosphere, he was able to create fire-resistant filaments for his incandescent light. In the 1950s, manufacturers began to realize the potential uses of the incredibly strong carbon fibers[10].
Buckminsterfullerene was discovered in 1965 by Kroto, Curl, and Smalley (see below). The soccer ball-shaped allotrope of carbon was named after R. Buckminster Fuller, an American engineer who designed the geodesic dome, which looked remarkably like a C60 molecule[7]. Research of C60 molecules led to the development of carbon nanotubes. In 1990, it was discovered that buckminsterfullerene could be produced in standard laboratories using an arc-evaporation apparatus. Using this same apparatus, Sumio Iijima discovered carbon nanotubes in 1991. Although these had been known about since the 1950s, Iijima's work produced higher quality nanotubes and sparked research into how to improve their structure. In 1993, single-layered nanotubes were developed[11].
A very rare form of carbon, white carbon, was first synthesized in 1969. Very little is currently known about this allotrope of carbon[12].
Occurrences

Carbon is found all throughout the universe in the sun, stars, comets, meteors, and the atmospheres of many planets (including earth's). In the sun (and other stars), carbon is produced through the process of fusion, in which helium nuclei fuse under intense heat and pressure to form carbon atoms[9]. Carbon is the fourth most abundant element in the universe, preceded only by hydrogen, helium, and oxygen[13].
On earth, carbon is the fourteenth most abundant element and is present in over one million organic compounds[9]. Natural gas and coal are made largely of carbon, the atmosphere contains carbon dioxide, and coral and seashells contain calcium carbonate [14]. It forms essential components of living organisms, such as DNA.
Carbon occurs naturally in several forms. Diamonds can be found in several places, most notably South Africa and also more recently on the sea floor off the Cape of Good Hope[15]. Graphite and amorphous carbon can also be found freely in nature. Rarely, lonsdaleite can be found at meteor landing sites[16]. Other allotropes of carbon do not occur naturally but can be created in a laboratory.
Carbon is found in all living organisms and it forms essential polymers, proteins, and carbohydrates that living organisms need[9]. Carbon makes up much of the food people eat as well. Carbohydrates, fats, proteins, and fiber all contain carbon compounds that are broken down by the body and used to repair tissues and synthesize chemicals. Carbon is also used for energy and carbon dioxide is given off as a waste product.
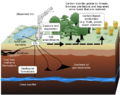
Carbon, which is present throughout the earth in various forms and compounds, moves and changes forms in the carbon cycle. In this cycle, carbon in the atmosphere (CO2) is absorbed by plants and used in photosynthesis. Some carbon also dissolves in the oceans and is absorbed by marine plants that also use it for photosynthesis[17]. Animals and humans obtain carbon in several ways. They eat plants, or eat other animals that ate plants, thus absorbing carbon through their food. Carbon dioxide is also a waste product of cellular respiration and is given off when animals exhale. In this way, the carbon is returned to the atmosphere. When animals die, their bodies decompose and the carbon enters the soil. Some of these dead organisms eventually become fossil fuels. Fossil fuels, such as coal and oil, release carbon back into the atmosphere when burned in the form of carbon dioxide gas. Through this whole process, carbon moves constantly between the atmosphere, plants, animals, and the soil and oceans as it changes form and is recycled[18]. This cycle is a closed cycle, meaning that there is a fixed amount of carbon in the earth that changes form but does not increase or decrease[17].
Carbon and Global Warming
Carbon often gets a bad name due to its role in global warming. Carbon dioxide is one of the greenhouse gases, meaning that it contributes to the greenhouse effect. Carbon dioxide, along with water vapor and other trace gases, reflects infrared radiation to trap heat from the sun within earth's atmosphere. However, this is a good thing--otherwise, the earth would be about zero degrees Fahrenheit! Thus, the greenhouse effect is necessary for life on earth. In addition, plants need carbon dioxide to live and perform photosynthesis[19].
Almost all scientists agree that global warming is occurring and that the temperature of the earth has risen slightly. Since the late 1800s, the earth's temperature has risen roughly 0.74 degrees Celsius[20]. One thing that not all scientists agree on is how much impact humans have on global warming. The causes of global warming are still not fully understood, and it results from a variety of factors. Water vapor, for example, not carbon dioxide, is the main greenhouse gas. In fact, if one takes water vapor into account, carbon dioxide makes up only about 0.28 percent of the earth's atmosphere (5.53 percent discounting water vapor)[21]. While carbon dioxide levels have certainly risen since the Industrial Revolution, man has not had as large of an impact on global temperatures as people think. An article by Answers in Genesis points out that the earth's climate has changed before. After Noah's flood, for example, the climate of the earth was significantly different and temperatures became much warmer. Later, however, when the earth entered an ice age, temperatures dropped dramatically. In medieval times the earth experienced another slightly warmer period, followed by a "little ice age" from 1300 to 1880 AD. These changes were due to environmental changes, including volcanic eruptions and fluctuations in the sun's radiation[22].
Additionally, the sun affects the earth's temperature much more than carbon dioxide levels do. The sun goes through cycles, such as the sunspot cycle. The amount of sunspots on the sun varies from few to many, increasing every eleven years and then decreasing again at the end of the cycle, and the amount of sunspots has been shown to affect the climate on earth. A greater amount of sunspots results in greater overall intensity to compensate for the dim regions, and this produces a higher temperature on earth. The sun is also believed to go through longer cycles, and it may have been one of these that caused the "little ice age"[19].
Global warming has been used, particularly in the political world, to create panic about terrible potential consequences. Extreme scenarios have been suggested, such as world-ending ice ages, disastrous storms and weather, and floods from rising oceans. However, these things are not currently as threatening as people are often led to think. According to the UN Intergovernmental Panel on Climate Change, oceans are only expected to rise about 16 inches this century. Also, no species have yet been recorded as extinct due to global warming[22].
Uses
Carbon is mined in many different forms. Carbonates such as limestone (calcium carbonate), dolomite, and marble are mined in large quantities, as are fossil fuels (coal, oil, and natural gas). These fossil fuels are a vital source of energy throughout the world.
Graphite can be mined and used for industrial purposes and in pencil "lead". A great conductor, graphite has many uses in electrochemistry. It is a component of batteries, fuel cells, and capacitors[23]. It is also often used as a lubricant due to its slippery nature[24]. Graphite can be produced from coke as well. Coke, a tar-like substance produced by burning liquid substances that contain carbon, is most often used in steel production[23].
Diamonds are highly valued for their beauty and are often used to make jewelry. They are also often used in industrial equipment such as saw blades and drill bits due to their incredible hardness.
Amorphous carbon is used in steelmaking, printing (carbon black), water treatment, and air filtration. Carbon black has long been used for writing purposes, but it now has many other uses as well. It is found in printers and photocopiers, plastics, and even rubber tires. In fact, about 90% of the carbon black produced is used by the rubber industry[23].
Carbon nanotubes are currently being researched for a wide range of applications, primarily in the mechanical, aerospace, and technological fields. Their strength, lightness, and conductivity make them extremely valuable in these fields[11]. Nanotubes are also used as biosensors and can be used in the medical field as implantable sensors that monitor things such as blood glucose levels and temperature[25].
Carbon fiber is used in fire-protective clothing, laminates for rockets and airplanes, and heavy-duty plastics for sports equipment[4].
The human body uses carbon for a variety of different purposes. Amino acids, for example, which form proteins, are made of functional carbon groups. Carbohydrates, which are important sources of energy for the body, are also carbon compounds[26].
Carbon compounds have many uses as well. Carbon dioxide, one of carbon's most well-known compounds, is used in things like carbonated drinks, fire extinguishers, and dry ice[27].
Carbon-14, an isotope of carbon, is used in carbon dating on fossils and minerals to find out how old they are. Scientists use the radioactive nature of carbon-14 to do this. Since carbon-14 has a half-life of 5,730 years (meaning half of it will decay in 5730 years), scientists measure how much carbon-14 is left in a given object and, based on the natural concentration of carbon-14 in the environment today, they estimate the age of the tested object[24]. Of course, this process can be inaccurate because scientists do not know the actual amount of carbon-14 that was originally in the object; they can only guess.
Allotropes
Diamond
Diamonds, one of the most well-known forms of carbon, are widely known for their beauty and hardness. In fact, they one of the hardest naturally occurring minerals. Although exceedingly strong, they can be split along four directions of cleavage. It also has a very high melting point at 3820 K. This strength is a result of the arrangement of the carbon atoms in a diamond; for one thing, they are packed very close together[28]. The carbon atoms are arranged in a tetrahedral way, so each carbon atom has four covalent bonds with other carbon atoms that are equidistant from each other. This structural arrangement makes diamonds strong and also very rigid. The functional unit of diamond is a cube with eight carbon atoms. These cubes form different shapes of diamonds, called crystal habits[29].
Diamonds, when polished, are transparent and lustrous. Their brilliance can be attributed to high light dispersion and a high refractive index. While they are usually clear, diamonds can be found in any number of colors, which are caused by impurities. Other properties include high thermal conductivity and negative electron affinity (they repel water). Diamonds are poor conductors of electricity, although some can be used as semiconductors, and they are chemically inert (do not react with most acids and bases)[29]. They are often used in jewelry, but they also have an important role in the industrial world. Due to their incredible hardness, diamonds are used for things such as drill bits, saw blades, and powder for polishing or grinding. Diamonds used for industrial purposes are usually not up to the standard for jewelry and are known as bort; these accounts for about 80% of mined diamonds[30].
Naturally occurring diamonds are classified in four categories based on their nitrogen concentration. They can be type Ia, Ib, IIa, or IIb based on their impurities and how much nitrogen they contain. Diamonds can also be synthesized in a laboratory. This method was first developed in the 1950s[30]. They are produced through High Pressure High Temperature Synthesis; graphite and a metallic catalyst are exposed to high pressure and temperatures, causing the graphite to change into diamond. Thin diamond coatings can be made through Chemical Vapor Deposition. These coatings can be put on machine parts to protect them from wear[29].
Graphite
Graphite, in contrast to diamond, is soft, opaque mineral with a dull black color. Although graphite and diamonds are made of the same element, their different atom arrangements give them their vastly different properties. In graphite, the carbon atoms are hexagonally arranged and form sheets that have only weak bonds to each other and can be perfectly cleaved. This means that graphite can be easily broken along its layers, and its slippery feel makes it useful as a solid lubricant. Graphite is graded as flaky, crystalline (lumpy), or cryptocrystalline (amorphous).
Graphite is often found in veins and pockets in other rocks and minerals, especially felspar, mica, quartz, pyroxene, rutile, pyrite, apatite, shale, slate, sandstone, and limestone. Deposits of graphite can be found in countries all over the world. Graphite can also be artificially produced from coal or coke[31].
Amorphous Carbon
Amorphous carbon is an allotrope of carbon that does not have a crystalline structure and is often found as a powder. Its atoms have only short-range order, with no long-range patterns. They often contains crystals of diamond or graphite and impurities of other elements. Coal is graded based on how much carbon it contains; coal with a higher percentage of carbon has a higher grade[30]. Amorphous carbon can be formed by heating carbon-containing substances to 1200-1800 degrees Fahrenheit in an atmosphere with limited oxygen (this prevents the substance from completely combusting). Kinds of amorphous carbon include coal, soot, carbon black (also called lamp black or channel black), coke, charcoal, bone char, baked carbon, carbon arcs, and carbon electrodes[32].
White Carbon
White carbon (also called ceraphite), first discovered in 1969, remains a mysterious and little-known allotrope of carbon. It was discovered when graphite was heated at low pressure to a temperature of over 2500 K[12]. At that temperature, some of the graphite sublimated and tiny white crystals appeared around the edges of the graphite[33]. Its properties include birefringence (the ability to split a single beam of light) and transparency[12].
Fullerenes

Buckminsterfullerene (C60), also called fullerene or "buckyball", is an allotrope of carbon where the carbon atoms are arranged to form hollow spheres. These spheres have a diameter of only one billionth of a meter but, due to their stability, can withstand incredible forces. Other sizes of fullerines have been synthesized, including 32-atom "buckybabies" and massive 960-atom spheres, and uses for these fullerines are still being discovered[34]. Buckyballs are very darkly colored, appearing nearly black in their solid form and a deep red in solution (unlike diamonds and graphite, buckminsterfullerene can dissolve in organic solvents)[2].
Buckminsterfullerene was first discovered in 1985 by Harold Kroto at the University of Sussex and Robert Curl and Richard Smalley at Rice University. Kroto was researching interstellar matter and the formation of long chains of carbon atoms. Kroto joined Curl and Smalley at Rice University, where they studied the effects of carbon vaporization using Smalley's machine, which fired a laser at graphite to vaporize it, then used hydrogen gas to pump the carbon particles into a mass spectrometer. The process led to the formation of C60 molecules, which appeared extremely stable[35]. From their research, they proposed that the structure of C60 was similar to that of a soccer ball, which has a pattern of hexagonal and pentagonal patches. In buckminsterfullerene, each carbon atom has one double bond and two single bonds (four total bonds per atom) with the atoms around it, forming a sphere of carbon atoms. This theory was later proven using nuclear magnetic resonance spectroscopy and x-ray diffraction. This arrangement of atoms is what makes buckyballs so stable. Also, the hollow sphere shape allows atoms of other elements to be trapped inside of a buckyball. Scientists have found few applications for buckminsterfullerene so far, but it has shown some promise in the areas of reinforcing fibers and medicine [36].
Nanotubes
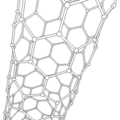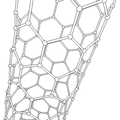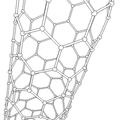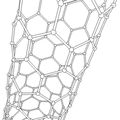
The atoms of nanotubes are arranged similarly to those in graphite, in layers of hexagonally arranged atoms. However, a key difference makes nanotubes much stronger than graphite. Instead of flat layers that lie on top of each other, the sheets of atoms are rolled into tubes. These incredibly tiny tubes, only a few nanometers in diameter, can reach a length of up to a millimeter[37]. Nanotubes contain two or more rolled layers of graphitic sheets, and they have closed ends. These are usually straight. Another class of nanotubes, which contain only one layer of atoms, are even smaller and are often curved[11].
Nanotubes can be produced using an arc-evaporation apparatus. This apparatus sends an arc of 50 volts through two graphite samples in helium gas, causing some of the graphite to evaporate and the condense again. The graphite that condenses on the cathode of the apparatus contains carbon nanotubes. Nanotubes can also be produced by catalytic synthesis, in which a gas that contains carbon is passed over a catalyst to produce the desired reaction[11].
Nanotubes have several properties that make them exceedingly valuable to the mechanical field. They are much stronger and also lighter than steel, with a tensile strength 50 times higher than steel. Different nanotubes may be conductors or semiconductors, depending on their structure[11].
Nanofoam
Nanofoam, a relatively recent discovery, was first developed at the Australian National University in Canberra in 1997[38]. Researchers placed a sample of graphite in a chamber filled with argon gas and fired a laser at the carbon sample at 76 million pulses per second until, at around 10,000 degrees Celsius, the carbon formed into clusters that interconnected randomly[39]. This nanofoam--a spongy, lightweight, porous, low-density solid--is a semiconductor and an insulator, able to withstand temperatures of thousands of degrees FahrenheitCite error: Invalid <ref> tag; invalid names, e.g. too many. Scientists are still exploring the possible uses of nanofoam, and many are hopeful that it will prove useful in the medical field. It could potentially aid in imaging blood flow in the body. Nanofoam could be injected into the bloodstream and, due to its magnetism, could be picked up by magnetic resonance imaging machines. Also, its property of insulation could help in treating tumors. If injected into a tumor, the nanofoam could absorb and trap infrared radiation fired at the tumor, thus killing the tumor while protecting the outside tissues. Of course, there could also be potential health hazards and risks associated with nanofoam; these have yet to be fully explored[40]
Lonsdaleite
Lonsdaleite, a naturally occurring but extremely rare allotrope of carbon, is 58% harder than diamond. Lonsdaleite is also known as the hexagonal diamond because of the hexagonal arrangement of the carbon atoms, which gives the substance its strength[41]. Lonsdaleite is translucent in appearance, and its color ranges from a black-brown to light yellow-brown. When it forms, it makes microscopic crystals[16].
Lonsdaleite is formed when meteors containing graphite fall to earth; the intense heat and pressure during the fall changes the graphite into a diamond-like substance with the carbon atoms arranged in a hexagonal lattice. This allotrope was first identified in 1967 in the Canyon Diablo meteorite in Arizona. It has also been found in the Goalpara meteorite, Kenna meteorite (New Mexico), the Allan Hills 77283 site, and the Tunguska impact site (Russia)[16][30].
Compounds
Carbon compounds are abundant all over the earth; in fact, scientists know about nearly 10 million carbon compounds[42]! One reason carbon can form so many compounds is that it has the ability to form extremely stable bonds with itself. Carbon atoms can form long chains or rings that are very stable. These provide the backbone for both organic and inorganic carbon compounds[43]. All of these compounds have some characteristics in common. For example, all organic carbon compounds will burn and can be used as fuel; carbon dioxide is produced in the reaction. Also, most carbon compounds cannot be dissolved in water[44]. A carbon atom always four covalent bonds, but these can be single, double, or triple bonds, allowing for a wide range of formations.[45].
Inorganic Carbon Compounds
It is commonly thought that organic compounds are those that contain carbon, and therefore all carbon compounds are organic. However, organic compounds can more accurately be described as compounds that contain both carbon and hydrogen. There are many inorganic compounds, including carbides, oxides, and carbonates.
When carbon reacts with a less electronegative element, a carbide results. These reactions occur at high temperatures. Carbides are classified as covalent, ionic, or interstitial. In covalent carbides, carbon reacts with an element of the same size and electronegativity (example: silicon carbide). Covalent carbides have properties similar to diamond in that they are very hard, are inert, and have high melting points. When carbon reacts with a relatively active metal, it produces an ionic carbide. These compounds contain negatively charged carbon ions, and they burst into flame when they come into contact with water. If carbon reacts with a metal of moderate electronegativity with a large atomic radius, it will produce an interstitial carbide. In this case, the carbon atoms exist in between the metal atoms, producing something similar to a metal alloy that still has most of the properties of the metal[46].
Oxides are another notable class of carbon compounds. These form at high temperatures, often in the presence of water or steam. The most important oxides are carbon monoxide (CO) and carbon dioxide (CO2). Carbon monoxide is dangerous because it bonds to the hemoglobin in the blood, preventing the body from absorbing oxygen. It can be toxic even in small amounts. Carbon dioxide is an important part of the atmosphere, but it can also be harmful in large amounts[46].
Carbonates, such as calcium carbonate, are found in the shells of many marine creatures, in egg shells, and in limestone. They are typically insoluble in water (although they will dissolve in carbonated water). Baking soda (sodium bicarbonate, NaHCO3) or bicarbonate of soda is another significant carbonate frequently used in baking[46].
Organic Carbon Compounds
Organic molecules are composed of a carbon backbone and a functional group. Carbon forms a perfect backbone because of the very stable chains and rings that it can form. Molecules with the same functional groups tend to have similar properties, so organic molecules are classified based on their functional groups. These functional groups include amino groups, alcohol groups, aldehyde groups, carboxyl groups, ester groups, keto groups, methyl groups, and phosphate groups[47].
Hydrocarbons, the simplest carbon compounds, are composed of carbon atoms and hydrogen atoms. Methane (CH4), ethane (CdH6), propane (C3H8), butane (C4H10), pentane (C5H12), and hexane (C6H14) are all hydrocarbons. These hydrocarbons are called alkanes because they have all single bonds. Alkenes have at least one double bond, such as ethylene (C2H4). An alkyne, like acetylene (C2H2), has at least one triple bond. Hydrocarbons are also classified as saturated or unsaturated based on their bonds. Saturated hydrocarbons have more hydrogen, converting their double and triple bonds to single bonds so that the molecule is in a straight line, while unsaturated hydrocarbons have less hydrogen and are bent. Hydrocarbons can also have ring structures that may contain double bonds, such as benzene (C6H6)[45]. Long chains of hydrocarbons form things such as gasoline and plastics[48].
Alcohols are essentially hydrocarbons where one of the hydrogens has been replaced with an -OH group. For example, methane plus oxygen becomes methanol. Alcohols are categorized based on the position of the -OH group in the molecule. Small alcohols dissolve easily in water, but as the hydrocarbon chains become longer, they grow more and more insoluble[49].
Carbohydrates, lipids, and proteins are carbon compounds that provide essential nutrients to the body. Carbohydrates contain carbon, hydrogen, and oxygen. They are composed of sugars, specifically monosaccharides and disaccharides that have bonded together. Lipids are made of fatty acids and do not dissolve in water. Both carbohydrates and lipids are used by the body to store energy. Proteins are essential to the body and are components of DNA. They are made of amino acids, which all contain an amino group and a carboxyl group. These functional groups join together through dehydration synthesis, which results in peptide bonds[50].
Visit Buzzle.com for a longer list of organic carbon compounds.
Gallery
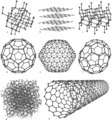
Eight Allotropes Of Carbon - a) Diamond, b) Graphite, c) Lonsdaleite, d) Buckminsterfullerene (C60), e) C540, f) C70, g) Amorphous carbon, h) single-walled carbon nanotube.
Carbon Nanotube
References
- ↑ 1.0 1.1 Carbon Chemicool Periodic Table, 16 Nov. 2010.
- ↑ 2.0 2.1 Buckyballs, Diamond, and Graphite: Introduction University of Wisconsin Department of Chemistry, 2010, 18 Nov. 2010.
- ↑ Carbon: the essentials Mark Winter, The University of Sheffield and WebElements, Ltd., 20 Nov. 2010.
- ↑ 4.0 4.1 4.2 4.3 Nature's building blocks: an A-Z guide to the elements John Emsley, Oxford University Press: 2001, pp 94-95.
- ↑ Carbon Isotopes Interactive Nano-Visualization in Science and Engineering Education, University of Arizona, 1 Dec. 2010.
- ↑ Isotopes of Carbon Gray Pilgrim, Buzzle.com, 11 May 2010, 1 Dec. 2010.
- ↑ 7.0 7.1 Carbon family Science Clarified, Advameg, Inc., 29 Nov. 2010.
- ↑ History of Carbon: Historical Production and Use of Carbon Materials University of Kentucky Center for Applied Energy Research, 17 Sep 2010, 2 Dec 2010.
- ↑ 9.0 9.1 9.2 9.3 The history and use of our earth's chemical elements: a reference guide Robert E. Krebs, Greenwood Press: 2006, second edition, pp 192-193.
- ↑ The History of Carbon Fiber Carbon Fiber Info, Illstreet.com, 2009, 2 Dec 2010.
- ↑ 11.0 11.1 11.2 11.3 11.4 A Carbon Nanotube Page Peter Harris, Centre for Advanced Microscopy at the University of Reading, 20 April 2010, 1 Dec. 2010.
- ↑ 12.0 12.1 12.2 Forms of Carbon John Blamire, BIOdotEDU, 2 Dec 2010.
- ↑ The Carbon Cycle Paul Przyborski and Lorraine Remer, The Earth Observatory, EOS Project Science Office, NASA Goddard Space Flight Center, 27 Nov. 2010, 30 Nov. 2010.
- ↑ carbon (c) Encyclopædia Britannica, 2010, Encyclopædia Britannica Online, 1 Dec 2010.
- ↑ CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data David R. Lide, CRC Press: 2004, 85th edition, pp 4-7.
- ↑ 16.0 16.1 16.2 Lonsdaleite Mineral Data David Barthelmy, Webmineral Mineralogy Database, 31 Dec. 2009, 22 Nov. 2010.
- ↑ 17.0 17.1 Carbon Cycle Chris Kreger and the ETE Team, Wheeling Jesuit University/NASA-supported Classroom of the Future, 10 Nov. 2004, 30 Nov. 2010.
- ↑ The Carbon Cycle Roberta Johnson, Windows to the Universe, National Earth Science Teachers Association (NESTA), 7 Nov. 2010, 30 Nov. 2010.
- ↑ 19.0 19.1 Global Warming’s Solar Connection: The Science Jason Lisle, Answers in Genesis, 20 June 2010.
- ↑ Global Warming Frequently Asked Questions National Oceanic and Atmospheric Administration, National Climatic Data Center, U.S. Department of Commerce, 20 Aug 2008, 2 Dec 2010.
- ↑ Water Vapor Rules the Greenhouse System Monte Hieb, 10 Jan 2003, 2 Dec 2010.
- ↑ 22.0 22.1 Global Warming in Perspective Melinda Christian, Answers in Genesis, 25 Aug 2008, 2 Dec 2010.
- ↑ 23.0 23.1 23.2 ELECTROCHEMICAL USES OF CARBON Kim Kinoshita, Ed. Zoltan Nagy, Electrochemistry Encyclopedia, Ernest B. Yeager Center for Electrochemical Sciences (YCES) and the Chemical Engineering Department, Case Western Reserve University, Jan. 2001, 30 Nov. 2010.
- ↑ 24.0 24.1 The Element Carbon Steve Gagnon, Thomas Jefferson National Accelerator Facility - Office of Science Education, 16 Nov. 2010.
- ↑ Carbon Nanotubes: Next Generation of Electronic Materials Jaldappagari Seetharamappa, Shivaraj Yellappa, and Francis D’Souza, The Electrochemical Society Interface, 2006, 1 Dec. 2010.
- ↑ Carbon-based Compounds and Functional Groups J. Stein Carter, 2 Nov 2004, 1 Dec 2010.
- ↑ Uses of Carbon Rahul Thudani, Buzzle.com, 30 Nov. 2010.
- ↑ The Mineral Diamond Amethyst Galleries, Inc., 2 Dec 2010.
- ↑ 29.0 29.1 29.2 Chemistry of Diamond Anne Marie Helmenstine, About.com, The New York Times Company, 2 Dec 2010.
- ↑ 30.0 30.1 30.2 30.3 Carbon Allotropes by NanoScienceWorks, supported by Taylor & Francis Group, LLC, 18 Nov. 2010.
- ↑ Graphite Mineralszone.com, 2005, 1 Dec. 2010.
- ↑ Properties of Carbon Leallyn B. Clapp, Dendritics, Inc., originally from Compton's Encyclopedia, 2 Dec 2010.
- ↑ White Carbon Shen, Everything2.com, The Everything Development Company, 14 May 2005, 2 Dec 2010.
- ↑ Carbon Cages: LBL Scientists Study Fullerenes Lynn Yarris, 1993, 20 Nov. 2010.
- ↑ Chemical of the Week: Buckyballs Bassam Z. Shakhashiri, Scifun.org, 16 Nov. 2010.
- ↑ Buckyball, Diamond, and Graphite: Buckminsterfullerene University of Wisconsin Department of Chemistry, 2010, 18 Nov. 2010.
- ↑ The Nanotube Site David Tomanek, Michigan State University, 1 Dec. 2010.
- ↑ What is Carbon Nanofoam? Michael Anissimov, Ed. R. Kayne, wiseGEEK, 3 Aug. 2010, 20 Nov. 2010.
- ↑ Crazy carbon nanofoam loves magnets Anna Salleh, ABC Science Online, 25 March 2004, 20 Nov. 2010
- ↑ Scientists create fifth form of carbon Jim Giles, Nature Publishing Group, Macmillan Publishers Ltd., 23 March 2004, 18 Nov. 2010.
- ↑ Diamond no longer nature's hardest material Jessica Griggs, NewScientist.com, Reed Business Information Ltd., 16 Feb. 2009, 22 Nov. 2010.
- ↑ Carbon Los Alamos National Labs Chemistry Division, University of California for the U.S. Department of Energy, 15 Dec. 2003, from the CRC Handbook of Chemistry and Physics and the American Chemical Society.
- ↑ Inorganic Carbon Compounds Encyclopedia about Chemistry, KENAX Translation Agency, 2 Dec 2010.
- ↑ Carbon Compounds Andy May, University of Bristol School of Chemistry, 1 Dec 2010.
- ↑ 45.0 45.1 Hydrocarbons CliffsNotes.com, 1 Dec 2010.
- ↑ 46.0 46.1 46.2 The Inorganic Chemistry of Carbon Bodner Research Web, The Bodner Group, Division of Chemistry Education, Purdue University, 2 Dec 2010.
- ↑ The Guide: Organic compounds and the importance of carbon 2 Dec 2010.
- ↑ C6: The Building Blocks of Organic Compounds chemistryland.com, 14 Sep 2008, 2 Dec 2010.
- ↑ Introducing Alcohols Jim Clark, Chemguide, 2003, 2 Dec 2010.
- ↑ The Guide: Different types of organic compounds 2 Dec 2010.
Additional Information
- What is the Carbon Cycle?
- Carbon MathMol, NYU/ACF Scientific Visualization laboratory.
- Chemical Element.com - Carbon Yinon Bentor, 2009.
- What is Carbon? S.E. Smith, Ed. Bronwyn Harris, wiseGEEK, 26 Oct. 2010.
- Facts About Carbon Facts About the Elements, 18 Nov. 2010.
- Carbon Cycle: Element Carbon CarbonCycle.biz, 2010.
- What is an Allotrope? Michael Anissimov, Ed. R. Kayne, wiseGeek, 10 Nov. 2010.
- The Mysterious Allotropes of Carbon John Borchard and Dendritics Inc., 2010, 18 Nov. 2010.
- Carbon The Third Millennium Online, 18 Nov. 2010.
- Carbon Compounds, Organics, and Organic Reagents
- Isotopes of Carbon Mark Winter, The University of Sheffield and WebElements Ltd, UK, 2 Dec 2010.
- Periodic Table of Elements- (C) Carbon
| ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||