Amino acid

Amino acids are organic molecules that contains amine and carboxyl functional groups. They are the basic building blocks used to make proteins, and are therefore a core component of living organisms. There are twenty different amino acids that are used to form proteins, which are first linked together to form chains called peptides. Peptides are then folded and joined with other peptides to form proteins, which are the machinery that perform the processes required by life.[1]
Structure
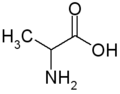
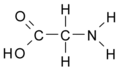
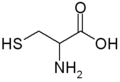
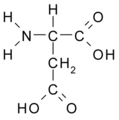
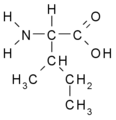
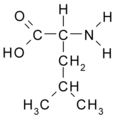
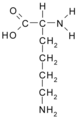
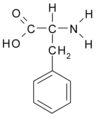
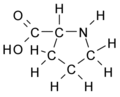
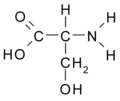
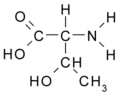
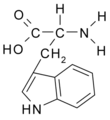
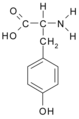
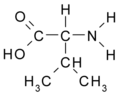
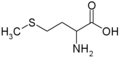
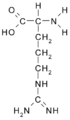
All amino acid molecules have an amine group (NH2 and the carboxyl group (COOH) (pictured at right). The individual amino acids each have a unique side chain (designated by the letter R). A side chain is a molecule that is attached to a core structure.
Amino acids are grouped according to the properties of their side chains because it is the side chain that determines the properties of the molecule. Some amino acids are weak acids, weak bases, hydrophilic, polar, or hydrophobic. [2]
Chirality
- Main Article: Chirality
Amino acids are chiral molecules, meaning that they exist as optical isomers of each other. The two forms are known as the D and L groups. A "chiral" molecule is one that cannot be superimposed on its mirror image. Just as our left and right hands are mirror images and not the same, chiral molecules have the same units attached in the same order, but as mirror images of each other.
Although most amino acids can exist in both left and right handed forms, life on Earth is made almost exclusively of only left handed amino acids. The L amino group is present in most amino acids that are found in proteins. The D amino group is found only in some proteins that are formed by exotic sea dwelling organisms. No one knows why this is the case, but it offers strong evidence that life was designed rather than the result of random chemical evolution.
Classification
Hydrophilic and Hydrophobic Amino Acids
A hydrophilic amino acid is one that attracts water. The hydrophilic amino acid has polar side chains that form weak hydrogen bonds with water and other polar or charged substances. A hydrophobic amino acid is one that is uncharged and non-polar and tends to repel water. The hydrophobic amino acid has a side chain that is made up of non-polar hydrocarbons or very slightly modified hydrocarbons. There are three different hydrophobic side chains: cysteine, glycine, and proline. The cysteine side chain can react with another cysteine chain to form a disulfide bridge, which is a covalent bond. A glycine side chain has a single hydrogen atom and is small enough to fit into tight corners in the interior of a protein molecule. It will fit into spots which would not accommodate a larger chain. A proline side chain differs from the other two types in the way it possesses a modified amino group. It lacks a hydrogen in its nitrogen, which limits its hydrogen bonding ability. Amino acids can be either hydrophilic or hydrophobic to some degree. This depends on the polarity of the side chain. Whether the amino acid is hydrophilic or hydrophobic determines how the amino acid will interact with other structures. This will determine the tertiary structure of the protein. The location on the outside determines the quaternary structure. [3]
Essential and Nonessential
Of the twenty amino acids, a few are called the essential amino acids because they are necessary for survival and cannot be synthesized de novo by the organism. They must be obtained from food. Histidine and arginine are two amino acids that are only used in children because these acids are not fully developed when they are in children. Histidine is used in the growth and repair of tissues. Some of the nutritionally important amino acids are Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine, Tryptophan, and Valine. If the body did not have Isoleucine it would then cause the body to have urinary problems. Threonine helps in the break down of uric acids with in the body. Valine is a major help for the development of body tissues. These are just an explanation of a few essential amino acids that are used throughout the body.
Non-essential amino acids are those that can be synthesized by the organism. Some examples of those are Alanine, Arginine, Asparagine, Asparatic acid, Glutamic acid, Glutamine, Glycine, Proline, Serine, and Taurine. Arginine is an amino acid that plays a role in healing wounds by removing ammonia from the body, and in the release of hormones. The nervous system uses Asparagines in order to maintain its equilibrium and also in the transformation of amino acids. Glutamine is used in the enhancement of muscles and is also involved with healing cell damage. [4]
Nonstandard
There are two nonstandard special amino acids, which are selenocysteine and pyrrolysine. A selenocysteine is present in several enzymes. They are not coded directly for genetic code unlike other amino acids. A pyrrolysine is an organism that is used to produce methane. These are not the only nonstandard amino acids there are actually many different ones that are not incorporated into a protein. Nonstandard amino acids are ones that occur mostly in the metabolic pathways of the twenty standard amino acids. These nonstandard amino acids are just modified versions of the twenty standard amino acids. [5]
Use in Proteins
- Main Article: Translation

Polypeptides (and eventually proteins) are created through the polymerization of amino acids. The product is the culmination of a complex series of chemical reactions collectively known as gene expression.
Amino acids are linked to one another during a condensation reaction that is catalyzed by an organelle called a ribosome. The ribosome performs the step in gene expression known as translation of the messenger RNA (mRNA). The mRNA is a copy of a gene, which is shuttled to a ribosome where the nucleotide sequence is read and the information used to assemble the polypeptide.
The mRNA is read 3 nucleotides at a time. This short sequences of nucleotides are referred to as codons. Each codon is coded information that the ribosome translates into an amino acid as it assembles the polypeptide.
Gallery
References
- Amino acid Wikipedia
- Amino Acids KWebMarketing
- Amino Acids University of Paisley
| ||||||||||||||