Nitrogen
| Nitrogen | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::N | ||||||||||||||||||||||||
| Atomic Number | Atomic number::7 | ||||||||||||||||||||||||
| Atomic Weight | Atomic weight::14.0067 g/mol | ||||||||||||||||||||||||
| Chemical series | Nonmetals | ||||||||||||||||||||||||
| Appearance | Colorless 
| ||||||||||||||||||||||||
| Group, Period, Block | 15, 2, p | ||||||||||||||||||||||||
| Electron configuration | 1s2, 2s2, 2p3 | ||||||||||||||||||||||||
| Electrons per shell | 2, 5 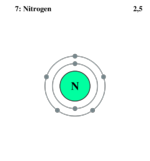
| ||||||||||||||||||||||||
| CAS number | CAS number::7727-37-9 | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| Phase | gas | ||||||||||||||||||||||||
| Density | Density::.0001251 g/ml | ||||||||||||||||||||||||
| Melting point | Melting point::63.15 K | ||||||||||||||||||||||||
| Boiling point | Boiling point::77.36 K | ||||||||||||||||||||||||
| Isotopes of Nitrogen | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||
Nitrogen is a colorless and odorless chemical element known by the atomic symbol N. It is one of the most essential elements to life and exists in about 78% of the earth's atmosphere. Nitrogen occurs in the amino acids, proteins, DNA, and RNA of humans. It is also very important to plant growth. Because it is often occurring naturally in unusable forms, it must react or be transformed into a usable substance. This occurs naturally when plants form it into a very useful aid in their growth. This is the factor that causes Nitrogen to be so bountiful in all types of fertilizers. Nitrogen has many other uses as well. It can be used as a medicinal applicant, anesthesia, and it is also used in tires of both airplanes and automobiles. Although nitrogen is essential for life, too much nitrogen can cause nitrogen narcosis, which is a state similar to intoxication. Overall, nitrogen can be considered a very innate gas, but obviously is used for many different things.
Properties
Nitrogen is a nonmetal or a chemical element. It is odorless and tasteless, so it often resembles water at its boiling point of -320.42F, because of its consistency and appearance as well. [1] Its gas form, or nitrogen gas, can be formed industrially by distillation of liquid air. It is said that nitrogen is the seventh most common element in the universe. By weight, even the human body is three percent nitrogen. This element is found all throughout the body's make-up and is central to all life and living organisms. [2]
Nitrogen is one of the elements that will not very willingly combine with many other elements. When nitrogen rarely bonds with oxygen, in the presence of some electrical force, it forms nitric oxide. As the simple element nitrogen is not a very active gas, God has created ways for plants to use it beneficially. The root hairs of plants hold bacteria that can chemically change nitrogen from the earth's crust into useful fertilizer. These now protein molecules aid in the growth of plants. [3] The element itself is found in the many building blocks of the body. Nitrogen is found in all different kinds of proteins and is essential to every aspect of life. It can be found also in DNA and RNA. [1]
Occurrences
Nitrogen has been considered very vital to life in many ways. Not only is it essential in the earth's atmosphere, making up 78% of it; this element also occurs naturally inside the human body. Nitrogen is a huge part of both animal, plant, and human tissues. Also, Nitrogen is, interestingly enough, a big part of the make-up of a human's genetic code. The Nitrogen molecules that occur outside are all a part of the "Nitrogen Cycle". These molecules can be found in the air and in the soil of the earth. The Nitrogen found in the soil, such at nitrates and nitrites, are very important in manures that are used for farming. Also, Nitrogen may be emitted by industries that, once they develop through the Nitrogen Cycle, may benefit the soil even more. [4]
Under the right circumstances, nitrogen may bond with oxygen to form nitrogen oxides. When it rains, these oxides dissolve and seep into the soil as nitrates. This is just a small part of the nitrogen cycle. In other circumstances, with extreme heat and pressure, hydrogen may bond with nitrogen to form ammonia. This substance can be used directly as fertilizer and is very beneficial to plants. These are two ways that nitrogen can be "fixed". If this element is not "fixed" then it cannot be used in the environment. Luckily, it can be naturally fixed so that it can be useful for the earth. [5]
Uses
Once nitrogen has combined with hydrogen to become the useful product, ammonia, it becomes one of the most important resources nitrogen produces. The ammonia industry is and has always been the larges consumer of nitrogen. These factories are major producers of fertilizer for plants and farms. Other industries, like the electronics industry, also use nitrogen. This industry uses nitrogen gas as a medium when making electrical components. This gas can also be used to anneal many steel products. This element can also be used to freeze or keep food cold while it is being transported. This is because of nitrogen's cold temperature. the liquid form of nitrogen is also used for many jobs. For example, it can be used to extract and bring oil up from wells and is also used in missiles.[6]
Because of its non reactive properties, Nitrogen also has many other useful aspects. Nitrogen is and can be used in many pharmacological and medicinal drugs. It can also be transformed into forms like nitrous oxide, which is used in hospitals as an anesthetic. Not only is this oxide used during surgeries, but also to preserve important biological things. Specimens like sperm, eggs, and embryos can be preserved by nitrous oxide. Computers and X-ray machines also use this gas. Tanks of nitrogen gas can be used very often. It often replaces carbon dioxide and is productively used in paintball guns and tires of all kinds. It can be found in situations where fire hazards could occur because nitrogen also combats fire. [7]
Nitrogen Narcosis
Nitrogen Narcosis is a serious and dangerous state that occurs when a human breathes nitrogen while under extreme pressure. This often occurs under pressure higher than normal atmospheric pressure. For example, this narcosis mostly occurs while deep-sea diving. Otherwise known as decompression sickness, this sickly state is very harmful to the body. The human brain's reasoning ability decreases by about a third, and the human body's dexterity also drops extremely. Just like the effects of drinking too much alcohol, nitrogen narcosis effects a person's motor skills and decision making, causing this state to be very dangerous for any diver. The ratio of nitrogen and other elements in the body are directly related to drunkenness as well as decompression sickness and the like. [8]
Nitrogen narcosis occurs when there is an unnecessary increased amount of dissolved nitrogen in the blood system. Although this seems extremely similar to decompression sickness, the two are still different. Breathing in extreme amounts of nitrogen causes a slight amnesia-like state, this is nitrogen narcosis. Decompression sickness, though, is caused by nitrogen bubbles slowly traveling into the diver's lungs from his blood. This is where the nitrogen is exhaled, causing decompression sickness. This is a very large difference between the two states. Also, while decompression sickness is caused strictly by the nitrogen bubbles forming as a diver descends, narcosis is caused by the depth of the diver. Decompression sickness may occur no matter how deep the diver swims. [9]
Video
10 different experiments to do with liquid nitrogen.
References
- ↑ 1.0 1.1 Facts About Nitrogen Live Science. Web. 15 April 2013 (published).
- ↑ Properties of Nitrogen. Boundless. Web. 21 October 2013 (accessed).
- ↑ Nitrogen. Chemistry Explained. Web. 7 October 2013 (accessed).
- ↑ Nitrogen-N. Lenntech. Web. 7 October 2013 (accessed).
- ↑ Nitrogen Cycle. Users.RCN.com. Web. 27 September 2013 (published).
- ↑ Visual Elements-Nitrogen. RSC. Web. 8 October 13 (accessed).
- ↑ Uses of Nitrogen. Uses Of. Web. 21 October 13 (accessed).
- ↑ Nitrogen Narcosis. Scuba-Doc.com. Web. 22 October 2013 (accessed).
- ↑ Gibb, Natalie. Decompression Sickness vs Nitrogen Narcosis - What's the Difference?. About.com. Web. 22 October 2013 (accessed).
| ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||



