Lanthanum
| Lanthanum | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::La | ||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::57 | ||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::138.90547 g/mol | ||||||||||||||||||||||||||||||
| Chemical series | Transition metals | ||||||||||||||||||||||||||||||
| Appearance | silvery-white metal 
| ||||||||||||||||||||||||||||||
| Group, Period, Block | 3, 6, D | ||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 5d1 6s2 | ||||||||||||||||||||||||||||||
| Electrons per shell | 2,8,18,18,9,2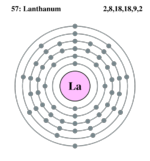
| ||||||||||||||||||||||||||||||
| CAS number | CAS number::7439-91-0 | ||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||
| Phase | Solid (at room temperature) | ||||||||||||||||||||||||||||||
| Density | Density::6.15 g/ml | ||||||||||||||||||||||||||||||
| Melting point | Melting point::918°C | ||||||||||||||||||||||||||||||
| Boiling point | Boiling point::3464°C | ||||||||||||||||||||||||||||||
| Isotopes of Lanthanum | |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||
Lanthanum is a chemical element classified as a Transition metals, and known by the chemical symbol La. Its name comes from the Greek word "lanthano," which means to "lie hidden". It is soft, silvery-gray, metallic solid that is very reactive. Lanthanum is found in the earth's crust and can be extracted from monazite and bastnaesite ores. Lanthanum can be used for many purposes, including the making of camera lenses, batteries, flint lighters, and carbon arc lamps.
Properties
Lanthanum is one of the transition metals.[1] At room temperature, it exists as a solid with a silvery-white color.[2] It possesses the properties of ductility and malleability, which means that it can be drawn into wires or molded into sheets.[1] Lanthanum is also extremely soft. In fact, it can be cut with a knife.[3] The structure of lanthanum changes as temperatures increase. At temperatures cooler than 310 degrees Celsius, it has a hexagonal structure, but as it warms up it becomes face-centered and cubic in structure. Once temperatures reach 865 degrees Celsius it changes into a body-centered cubic structure.[4]
Lanthanum is more reactive than most other rare elements.[1] When exposed, it reacts with the oxygen in the air, causing it to quickly oxidize. It even can react with cold water and form lanthanum hydroxide.[4] [5] However, the reaction is much more violent when the water is warmer. It also combines with several other elements such as carbon, nitrogen, boron, selenium, silicon, phosphorus, sulfur, and the halogens.[4] Lanthanum has a relatively low electronegativity of 1.1 on the Pauling's electronegativity scale and a first ionization energy of 538.1 kJ/mol, which is also quite low.[3][6]
Only two isotopes of lanthanum occur naturally. 99.91% of this element's atoms are lanthanum-139, the more common isotope.[2] The rest appears as lanthanum-138, a radioactive isotope with a half life of about 100 billion years.[7]
Occurrences
Lanthanum may be found in the earth's crust. It forms about thirty-four parts per million by weight or five parts per million by moles of the crust's total substance.[2] This makes it one of the more common rare earth elements.[8] Pure lanthanum does not typically exist in nature. However, it may be extracted from different ores.
Lanthanum is mostly found in two different minerals: monazite and bastnaesite.[2] Monazite is mined in several places including Australia, Brazil,India, Malaysia, South Africa, Sri Lanka, and Thailand. Sources of bastnaesite are located mainly in China and the United States.[9] Monazite contains 25% lanthanum and bastnaesite consists of about 38% lanthanum. Pure lanthanum can be isolated by a process in which anhydrous fluoride is reduced with calcium.[4]
Uses
Two compounds of lanthanum, lanthanum fluoride and lanthanum oxide, are utilized in carbon arc lamps.[1] These lamps provide lighting for studios and projectors.[10] They are also used to make phosphors, substances that emit light when struck by electrons. Phosphors create the colors in television sets and can also help lessen the danger of radiation in x-rays.[1][4] Certain types of glass, such as the kind used in camera lenses, contain lanthana, another lanthanum compound.[10] Lanthanum glass can be used to produce optical fibers, wire-like materials that can carry light to transmit messages.[1] Misch metal, which also contains lanthanum, is involved in the making of flint lighters.[10]
Lanthanum, when used as an additive to steel, can increase the metal's resistance and malleability. The zeolite catalysts used in petroleum refining contain lanthanum salts as a stabilizing agent.[8] Another application of lanthanum is in the batteries of hybrid automobiles. The negative electrode in these batteries contains several different metal hydrides, one of which is lanthanum hydride.[2]Lanthanum also composes part of the hydrogen sponge alloy in fuel cells, which stores 400 times its own volume in hydrogen.[5][4]
History
Lanthanum was first found in 1839 by Carl Mosander, a Swedish chemist. This took place at the Karolinska Institute in Stockholm while Mosander was studying a sample of cerium nitrate.[11][3]He observed that though most of the cerium nitrate was insoluble in nitric acid, part of it was soluble. Mosander separated the soluble substance from the rest of the cerium nitrate. He concluded that a new element must be present.[11]
Mosander called this new element lanthana. This term comes from the Greek word "lanthano", which means to "lie hidden".[2]. However, this "element" was actually a mixture of six new elements. Over a period of sixty years, scientists isolated these elements and separated them from each other. A pure sample of lanthanum was finally obtained in 1923.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Lanthanum (revised) encyclopedia.com. Web. Accessed on October 11, 2014. Author unknown.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Stewart, Doug. Lanthanum Element Facts. Chemicool.com. Web. Published October 17, 2012.
- ↑ 3.0 3.1 3.2 Winter, Mark. Lanthanum: the essentials. WebElements.com. Web. Accessed on October 11, 2014.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Lanthanum. Molycorp. Web. Accessed on October 11, 2014. Author unknown.
- ↑ 5.0 5.1 Lanthanum. lookchem.com. Web. Accessed on October 11, 2014. Author unknown.
- ↑ Batdorf, Brad., Santopietro, Rachel., Cox, Heather., Porch, Thomas., and Wetzel, John. Chemistry. Greenville, S.C: Bob Jones University Press, 2009. 115-117. Print.
- ↑ LANTHANUM . Chemistry explained: foundations and applications. Web. Accessed on October 12, 2014. Author unknown.
- ↑ 8.0 8.1 Lanthanum - La. Lenntech.com. Web. Accessed on October 12, 2014. Author unknown.
- ↑ Lanthanum. REEhandbook. Web. Accessed on October 12, 2014. Author unknown.
- ↑ 10.0 10.1 10.2 Gagnon, Steve. The Element Lanthanum Jefferson Lab.org. Web. Accessed on October 19, 2014.
- ↑ 11.0 11.1 Lanthanum Royal Society of Chemistry. Web. Accessed on October 19, 2014. Author unknown.
| ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||


