Mendelevium
| Mendelevium | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||
| General Info | |||||||||||||||||||
| Atomic Symbol | Atomic symbol::Md | ||||||||||||||||||
| Atomic Number | Atomic number::101 | ||||||||||||||||||
| Atomic Weight | Atomic weight::258 g/mol | ||||||||||||||||||
| Chemical series | Actinides | ||||||||||||||||||
| Appearance | Unknown | ||||||||||||||||||
| Group, Period, Block | n/a, 7, f | ||||||||||||||||||
| Electron configuration | [Rn] 5f13, 7s2 | ||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 31, 8, 2 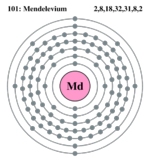
| ||||||||||||||||||
| CAS number | CAS number::7440-11-1 | ||||||||||||||||||
| Physical properties | |||||||||||||||||||
| Phase | Solid | ||||||||||||||||||
| Density | Density::Unknown g/ml | ||||||||||||||||||
| Melting point | Melting point::1100K | ||||||||||||||||||
| Boiling point | Boiling point::Unknown | ||||||||||||||||||
| Isotopes of Mendelevium | |||||||||||||||||||
| |||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||
Mendelevium (pronounced men-deh-LEE-vi-em) is a radioactive chemical element with the symbol Md and the atomic number 101. It is a metal, classified as a transitional element and a transuranic actinide, which is located in the bottom row of the periodic table. This element was discovered by the University of Berkeley, and named after Dimitri Mendeleev, who developed the Periodic Table.[1]
Properties
Mendelevium is a solid, which is synthetic or man-made. This element is synthesized bombarding other elements like Einsteinium with charge particles.[2]256Md has been used used to find some of the chemical properties in aqueous solution. This element has moderately stable dispositive oxidation state and can be shown to have a monopositive state. [1]
Discovery
Mendelevium was first discovered by Albert Ghiorso, Glenn T. Seaborg, Gregory R. Choppin, Bernard G. Harvey, and Stanley G. Thompson in 1955 at the University of California, Berkeley.it is named in honor of Dimitri Mendeleev who developed the Periodic Table of elements. Berkeley researchers produced 256Md when they after bombarding Es with alpha particles in the Radiation Laboratory's 60-inch cyclotron(a type of particle accelerator).[3]
Mendelevium Compound
The sections of binary compound with some compounds of mendelevium have a formal oxidation number for Mendelevium. However, uses of this compound limited for p-block element. Looking at oxidation number, and electronic configuration are not given. Exotic compound only view this as guide. In compounds of Mendelevium, the common oxidation number is three.
Hydrides •none listed
Fluorides •none listed
As a result, Mendelevium does not have compounds.[4]
Uses
Mendelevium does not exist in nature and was been produced in small amounts which are expensive and are time consuming to synthesize. It used for scientific research. [2]
Dmitri Mendeleev
Dimitri Mendeleev was born in Tobolsa, Samaria in 1834 and died in 1967. He arranged the elements into the periodic table based on atomic weight. His first periodic table was arranged by increasing atomic weight and grouping them by similarity of properties. However, he wasn't able to knew about noble gas element, which he does not included in the periodic table.[5]He studied thermal expansion which is the concept of changing matter and changing temperatures. At the end of his age, he retire from being college professor.[2]
Isotope
Mendelevium has eighteen radioisotopes. Md258 is the most stable isotopes and this isotopes bombardment of isotopes of element Einstein.
| Isotope | Atomic Mass | Half-life |
|---|---|---|
| Md245 | 245.08 | 0.90 ms |
| Md246 | 246.081 | 1 seconds |
| Md247 | 247.08 | 1.12 seconds |
| Md248 | 248.082 | 7 seconds |
| Md249 | 249.03 | 24 seconds |
| Md250 | 250.084 | 52 seconds |
| Md251 | 251.084 | 4 minutes |
| Md252 | 252.086 | 2.3 minutes |
| Md253 | 253.087 | 12 minutes |
| Md254 | 254.089 | 10 minutes |
| Md255 | 255.091 | 27 minutes |
| Md256 | 256.094 | 77 minutes |
| Md257 | 257.095 | 5.52 hours |
| Md258 | 258.098 | 51.5 days |
| Md258m | 57 minutes | |
| Md259 | 259.1 | 1.6 hours |
| Md260 | 260.1 | 31.8 days |
| Md261 | 261.1 | ~40 minutes |
| Md262 | 262.1 | ~3 minutes |
Video
References
- ↑ 1.0 1.1 Mendelevium. Wikipedia. Web. Accessed November 3, 2011.
- ↑ 2.0 2.1 2.2 Author-Unknown. What is Mendelevium. Wisegreek. Web. © 2003 - 2011.
- ↑ Author-Marion Coon Marion. Mendelivium. The Encyclopedia Of Earth. Web. November 24, 2009, 9:05 pm.
- ↑ Author-Markwinter. Mendelevium Compounds. Web Elements. Web. © 1993-2011.
- ↑ Author-Unknown. Dimitri Mendeleev. KIwi-web. Web. � HA Campbell 1998-2011.
- ↑ Author-Unknown. Isotopes. Unknown. Web. access 17 November 2011.
| ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||

