Iron
| Iron | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||
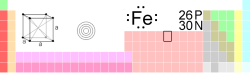
| Atomic Symbol | Atomic symbol::Fe | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::26 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::55.845 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Transition Metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | lustrous, silver-gray, metallic 
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 8, 4, d | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 4s2 3d6 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 14, 2 
| ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7439-89-6 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Density | [[Density::7.874 g·cm−3 g/ml]] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::1811 K | ||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::3134 K | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Iron | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||
Iron is a chemical element with an atomic number of 26. It is a transition metal, and one of the few ferromagnetic elements. It is extremely abundant in the earth's crust and commonly found in certain types of meteorites. Iron is known for its tendency to oxidize, or rust, when exposed to air or water. It is mined heavily all over the world. The majority of all iron mined is for the production of steel. The U.S. is one of the biggest producers of iron.
Properties
Iron is most commonly known as a metal. It is lustrous(shiny), and is a very good thermal and electrical conductor. It is malleable, meaning it can be hammered and formed into different shapes, and it is ductile, meaning it can be stretched into wire. Iron can be attracted by a magnetic substance, and can, itself, attract other metals. It can exist in four different crystalline forms, the most common of which is called Ferrite and has a body centered, cubic structure. [1] Between 1185-1667 K, the structure becomes face-centered cubic. it has an electronegativity of 1.83 (Pauling scale). The atomic radius of an iron atom is 140 pm (picometer=one trillionth of a meter). It has a hardness of 4.0 on the Mohs scale. [2] Iron has a specific gravity of 7.874. Pure iron is is reactive and allotropic, meaning it can react as both an acid and a base. When exposed unprotected to moisture or air it will oxidize(rust) and start corroding quickly, exposing the fresh surfaces beneath to oxidation, until the entire quantity of iron has been corroded away. [3]
Occurrences
Iron is an abundant metal. It can be found in many places on earth and throughout the universe. It is believed that the earth's core is made up of a solid combination of iron and nickel. Iron is also frequently found in meteorites, in large or small quantities. A type of meteorite called siderites largely contains iron. It is also present in the sun. Iron in the earth's crust is usually in ores. Hematite is the most abundant ore and is what we separate usable iron metal from. Black sands on beaches and stream banks are made up of minerals which contain iron (taconite & magnetite). [4] Mars contains a large amount of iron oxides in it's ground, giving the land its rust-like red appearance. It is believed that the earth's contains about 230 billion tons of pure iron, within the some 800 billion tons of iron ores. The U.S. produces about 27 billion tons of iron; the other biggest producers are Russia, Brazil, China, Australia, and India. [5]
Uses
Iron is a very important mineral in the human body, as well as living organisms in general. It is present in hemoglobin, which is the oxygen-carrying molecule in red blood cells that gives them their red color (when oxidized a.k.a. exposed to oxygen) and transports oxygen to the tissues.
Pig iron, which is mixed with a little carbon and very small amounts of silicon, phosphorus, sulfur, and manganese, is the prominent ingredient in steel, and other alloys. Steel is probably the most important iron-based product in the world, most conveniently since iron is the least expensive and most available of all metals. Wrought iron, having much less carbon, is much stronger than pig iron; it is also malleable where Pig iron is brittle. Wrought iron is not used in the making of alloys as it does not fuse well. [6]
Most iron is mined solely for the purpose of making steel. When the raw iron is combined with other elements in the process of making steel, it becomes stronger and less likely to oxidize. Steel is high necessary in the areas of construction and transportation. Powdered iron is used in magnets and auto parts. Iron 59, a radioactive isotope, is used in some medicines. [7]
Magnet
An iron ore called Magnetite can be magnetic, while other ores, such as Hematite, are not. Lightweight amounts of magnetic material, when suspended, tend to always point North and South. The early Chinese were the first to make use of magnetic iron to create compasses, which they had been using for over a thousand years before the Europeans ever discovered them. Scientists began to notice a kind of field around magnets. When you put a bar magnet under a sheet of glass and sprinkle iron dust over the glass, the dust forms the shape of arcs from end to end of the magnet. This field is responsible for the repulsion between certain sides of two magnets. Magnets have two distinct "poles", a North and a South, just like the earth itself. When the North of one magnet attracts the South of another, the magnetic field flows through the both of them as if they were one whole magnet, bending into the South end of the bottom magnet and coming out of the North end of the top magnet. When the same poles (North for example) of two magnets are forced together, the opposite flows of magnetic energy repel each other, pushing them apart. In iron, groups of atoms, called domains, work like tiny individual magnets, lining their poles up North to South, and interlock giving the total material one mutual magnetic field. [8]
References
- Iron:Properties The Columbia Electronic Encyclopedia, 6th ed. Copyright © 2007, Columbia University Press. Infoplease.com
- Iron (Ferrum) Chemical Properties & Compounds Author unknown. www.chemistry.patent-invent.com
- Iron Facts Chemical & Physical Properties By Anne Marie Helmenstine, Ph.D. About.com
- IRON ORE: Hematite, Magnetite & Taconite Mineral Information Institute. www.mii.org
- Magnetism StarGazers. NASA. nasa.gov
| ||||||||||||||



