Promethium
| Promethium | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Pm | ||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::61 | ||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::145 g/mol | ||||||||||||||||||||||||||||||
| Chemical series | Lanthanides | ||||||||||||||||||||||||||||||
| Appearance | luminescent in the dark with a pale blue or greenish glow 
| ||||||||||||||||||||||||||||||
| Group, Period, Block | Lanthanides, 8, F | ||||||||||||||||||||||||||||||
| Electron configuration | [Xe]4f56s2 | ||||||||||||||||||||||||||||||
| Electrons per shell | 2,8,18,23,8,2 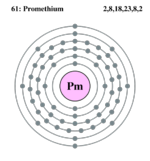
| ||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-12-2 | ||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||
| Density | Density::7.26 g/cm g/ml | ||||||||||||||||||||||||||||||
| Melting point | Melting point::1042°C | ||||||||||||||||||||||||||||||
| Boiling point | Boiling point::3000°C | ||||||||||||||||||||||||||||||
| Isotopes of Promethium | |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||
Promethium is a chemical element known by the symbol Pm. It has a distinct appearance with a luminescent color that shows off a bluish greenish glow without giving off heat.
Even though scientists have searched for years to find Promethium naturally, it has never been done; instead, it has been artificially manufactured. Several claims of discovering element number 61 were made before the actual discovery. It was really discovered by scientists at Oak Ridge Laboratory in Oak Ridge, Tennessee. It has been spotted in the spectra of some specific stars from the galaxy Andromeda.
Promethium is used to make certain types of batteries that are too expensive for the average user. These batteries are most widely used in space probes and satellites because of the demand for lightweight energy. It is also used for thickness gages, generally in metal sheets.
Properties
Promethium has soft beta emitter, although, it does not emit gamma rays X-radiation, or X-rays, can be formed when beta particles collide with the elements of another element with a high atomic number. If great care isn't taken, the right outcome will not be satisfactory. Promethium's greatest and most distinct physical property is its luminescence; giving off light without giving off heat. Fireflies' light is one example of the luminescent color. Promethium's salts bring attention to every eye in a dim or dark room with a pale blue or greenish glow, because of its high radioactivity. Ion-exchange methods brought scientists to the preparation of about ten grams of Promethium from atomic reactor fuel, which processes wastes, in early 1963. Not much has been discovered about the properties of metallic Promethium, but two allotropic modification do in fact exist. [1]
Occurrences
Though scientists and chemists have searched long and hard throughout the years, Promethium has yet to be discovered in the earth's crust; although, in the spectra of some stars in the galaxy of Andromeda, Promethium has been observed. This has most-likely happened because of nuclear reaction in other stars. The spectrum of a star is the light that is given off by the star, which is caused by Promethium. [2]
Since Promethium has yet to be discovered by natural means, it has been made artificially. Because of its specialized and unique ways to us it and its rarity, average customers do generally use Promethium. [3]
Uses
If Promethium is absorbed by a phosphor, it will produce light. If light is generated in this form and/or manner, it can be used for signs or signals that need to run perfectly for them to be useful. It can also be used in nuclear-powered batteries by capturing its light with photocells, which transform it into an electric current. A battery made from these steps could last up to five years of usefulness, using 147Pm. Promethium could become a reliable source for portable X-rays. It may one day even be used as a heat source to provide auxiliary power for all the space probes and satellites out there. The battery made from Promethium is one that can be put in places where other batteries of this power would be too heavy, such as the space probes and satellites. Over thirty Promethium compounds have been prepared and most of these are colored. [4]
As you can see, Promethium's uses are limited; it's best use is power from the radiation if gives off. These batteries, however, would be far to expensive to use in the common home. For example, lets say that thin sheets of metal are forming on a conveyor belt. If a sample of promethium metal is set above the metal and a detector is placed below, then the detector will count the amount of radiation that is flowing through the metal. As the piece of metal thickens, less radiation comes through, but as the metal slims, more radiation is able to go through. Then the detector records where the metal is too thin, and where the metal is too thick. The detector can also stop the conveyor belt when this happens. [5]
Discovery and naming
Russian chemist, Dimitri Mendeleev, discovered the periodic table in the late 1860s.[6] The periodic table of elements is used to organize the elements into certain categories based on properties they share in common. The consists of eighteen columns and seven rows; each element has its own box of information.
By the time 1900 hit, most elements on the periodic table had been discovered, but just a few slots were still open. In 1902, Czech chemist Bohuslav Brauner hypothesized that there should be an element between Neodymium and Samarium (numbers 60 and 62). So chemists began their work.[7]
Italian chemists Luigi Rolla and Rita Brunetti said they found the element that would occupy number 61. They wanted to name it florentium after Florence, their home town. Around the same year or so, some scientists and the University of Illinois also claimed to have discovered element number 61. They also wanted to name it illinium after their home town of Illinois.
After some studies on element number 61, scientists began to believe it was radioactive; a radio active element breaks apart to give off some form of radiation. In the late 1930s, some more scientists at Ohio State University thought that they had now found element number 61. They wanted to name it cyclonium after the kind of particle accelerator they used to make the element.
Finally, the problem of whether or not any of these guesses were true or not was solved. During World War II (1939-45), scientists at Oak Ridge Laboratory in Oak Ridge, Tennessee, were studding the materials formed during atomic fission; the process in which large atoms are broken apart, which releases large quantities of energy with smaller atoms. These atoms are called fission products. Oak Ridge scientists proved that element number 61 was present in fission products of Uranium. They named this element Promethium, after the Greek god Prometheus, who, as legend states, stole fire from the gods and brought it down to earth for man to use.[8]
References
- Promethium University of California, 12/15/2003.
- PROMETHIUM Chemistry Explained.
- Promethium
- What is Promethium? S.E. Smith, conjecture corporation, 2003-2009.
| ||||||||||||||
