Astatine
| Astatine | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

| |||||||||||||||||||
| General Info | |||||||||||||||||||
| Atomic Symbol | Atomic symbol::At | ||||||||||||||||||
| Atomic Number | Atomic number::85 | ||||||||||||||||||
| Atomic Weight | Atomic weight::210 g/mol | ||||||||||||||||||
| Chemical series | Halogens | ||||||||||||||||||
| Appearance | Metallic 
| ||||||||||||||||||
| Group, Period, Block | 17,6,p | ||||||||||||||||||
| Electron configuration | [Xe] 6s2, 4f14, 5d10, 6p5 | ||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 7 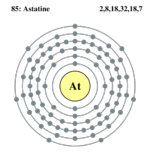
| ||||||||||||||||||
| CAS number | CAS number::7440-68-8 | ||||||||||||||||||
| Physical properties | |||||||||||||||||||
| Phase | solid | ||||||||||||||||||
| Density | Density::7 g/ml | ||||||||||||||||||
| Melting point | Melting point::575 K | ||||||||||||||||||
| Boiling point | Boiling point::503 K | ||||||||||||||||||
| Isotopes of Astatine | |||||||||||||||||||
| |||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||
Astatine is the last know Halogen with an atomic number of 85. The element is the rarest natural occurring of all of the 118 known elements. When Astatine was discovered the scientists named it Astatine which came from the Greek word astatos, meaning unstable. The element received the name because the longest half-life of its longest isotope is just barely over seven hours. This fact makes it almost impossible to find any common, and efficient uses for Astatine. However, there are theories about using Astatine to fight cancer. Of the halogens Astatine is the least reactive, and it behaves much closer to a metal than the other halogens. There is only a few elements that Astatine will bond with, and of these resulting compounds none of them will last very long, before the Astatine decays away.
Properties
Since Astatine has an incredibly short half-life there are little details about its chemical properties. Scientists at the Argonne National Laboratory performed detailed studies in the short time that the element existed in 1996. They found that the element behaves like Iodine, but has more metallic properties.[1] Since both Astatine and Iodine are Halogens they would share some of the same properties, because they each have seven valence electrons. Valence electrons determine how an atom will bond, because the ultimate purpose of atomic bonding is to achieve a full outer shell of electrons. However, difficulty in determining Astatine's properties is further compounded by impurities in the samples. When Astatine bonds its resulting compounds will not last very long, due to Astatine's short half-life.[2] When Astatine bonded with Hydrogen it produced an acid called Astatane. This is the most powerful of all of the acids, but one of the least useful. The acid will readily decompose back into its elemental forms when Astatine breaks down into Bismuth, or Polonium. This happens because the radioactivity of Astatine is too great to allow any long lasting compounds.[3]
A time of flight mass spectrometer is used to measure some of Astatine's properties because it decays incredibly fast. The spectrometer uses a fixed distance ratio, and calculate the size of the particle passing through it based on the speed which it takes for the particle to completely travel through the device. This device was used to measure the size of Astatine, and other heavy elements. [4]
Occurrences
Astatine can be found in small amounts naturally in nature, but the majority of the Astatine used is produced synthetically in laboratories.
Natural
Astatine occurs in very small amounts throughout the world. Astatine's half-life is incredibly short, around seven hours, the amounts present in the world are very low. It is thought that there are never more that 30 grams of it present at any given time in the world. When Thorium and Uranium decay, they will eventually decay into Astatine. The only natural occurring samples found to date, occur in trace amounts present in samples of Uranium and Thorium. [5]
Synthetic
In the 1940's in the University of California, the isotope Bismuth-209 of the element bismuth was collided with Alpha Particles. This test produced Astatine for the very first time in a laboratory. Since that little amounts of Astatine have been produced, due to its short half[life and high radioactivity. The Astatine that has been produced does not last long, and because it is fairly costly too manufacture, most labs do not bother to manufacture it. [5]
Uses
Currently there are no widespread uses for Astatine, due to its high radioactivity. However there is speculation of possibly using it as a possible cancer treatment. Cancer cells are killed off when exposed to radiation, and this process is called radiation therapy. Astatine would probably work best to treat cancer cells in the thyroid gland, located at the base of the neck. Since many of Astatine's properties are similar to those of iodine, which accumulates in the thyroid, Astatine would be injected into the human body and it would make its way to the thyroid where its radiation would kill the cancer cells. [6] Other possible uses of Astatine include using the alpha particles it emits to target single cancer cells. Astatine decays very quickly and during its decay process it produces alpha particles, two protons and two neutrons bound together, and these particles when directed precisely have to potential to kill cancer cells. Currently this process uses beta particles, and while it has had a little affect it is not very practical. Because Beta particles are tiny, single electrons, and have extremely high speeds, it is difficult to get the required amount, over a thousand, to hit the cancer cell. In comparison the alpha particles produced by Astatine have the potential to kill the cancer cell with a single direct hit. Although this theory may work later in the future as of now it has a major problem. The half-life of Astatine's longest isotope is around seven hours so the element would have to be produced, and shipped to the testing sight, in a matter of hours. This problem cannot be corrected at this time, because the technology does not exist to stabilize the isotope.[7]
Radioactivity
Radioactivity is a process where elements, and isotopes lose part of their atomic structure through instability in the nucleus. There are three main branches of radioactive decay, and they are Alpha decay, Beta decay, and Gamma decay. Each of the three main areas of radioactive decay loses different particles through their decay. The element Astatine is created when uranium decays into Astatine. Then Astatine decays into Polonium, and Bismuth. Astatine decays through three main types of Radioactive decay: Alpha, Beta, and Gamma.
Alpha Decay
Alpha decay is a process where the nucleus of an atom loses two protons, and two neutrons, a helium nucleus. These are massive particles that can deal a lot of damage when they hit a gene, or a particle, directly. However, they are one of the least deadly forms of radiation, because of their lack or penetrating power. Since they are extremely large in size they cannot penetrate very deeply into an object. The alpha particles can be stopped with something as thin as a piece of tissue paper. This lack of penetration potential means that the particle is easy to protect against. Alpha particles were what Rutherford used in his experiments in 1911, when he discovered the nucleus of the atom.[8]
Beta Decay
Beta decay is when a neutron changes into a proton, and electron. This change occurs when there is an unequal balance between the protons and neutrons in the nucleus. The instability becomes too great, and in order to keep the nucleus together, a neutron transforms into a proton and electron. The proton remains in the nucleus while the negatively charged electron is expelled from the nucleus at high speeds. This particle, called a Beta Particle, is unstable and it takes only a slight interaction with matter in order to cause the Beta particle to react with the substance. In terms of penetration the Beta particles are more dangerous than Alpha particles. Since the Beta particles have incredibly high speeds, and small size they will penetrate much further than the slower and larger alpha particles. However the Beta Particles can be stopped by a thin piece of metal, or similar material.[9]
Gamma Decay
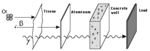
Gamma decay is produced from the nucleus of the atom moving to a lower energy state. Electrons do this whenever they are excited by heat, or other stimulus and the energy released is a couple of eV, electron volts. The eV is a unit of energy that is 1.60217646 × 10-19 joules, and is used to measure the energy of an electron. The energy that is released through the excited electron returning to its original position is released as light or heat. The energy can be seen and measured by utilizing a spectroscope or other similar devices. However when the nucleus shifts to a lower energy level large amounts of energy are released. Instead of being only a few eV’s emitted there is over 400 eV of energy emitted. This energy takes the form of a wave, and is called a gamma ray. Gamma rays are the most deadly form of radioactivity, and they have a very high amount of penetration. Only a few materials prove practical in stopping gamma rays, mainly lead, because they need high densities. Gamma rays can penetrate up to several feet of concrete, and when they approach a human body, there is little resistance and the ray can cause massive internal damage. [10]
References
- ↑ Astatine. Chemistry Explained: Foundations and Applications. Accessed November 13, 2011. Author unknown.
- ↑ The Halogens. chemwiki.ucdavis. published May 20, 2010. Author-unknown.
- ↑ Hydrogen astatide. Wikipedia/. published October, 31, 2011. Author-unknown
- ↑ Everett. ASTATINE. Mr T Everett. Accessed November 16, 2011.
- ↑ 5.0 5.1 The Element Astatine. It's Elemental: The Periodic Table of Elements. accessed 10-28-11. Author-unknown
- ↑ Astatine. The Periodic Table of the Elements. Accessed November 3, 2011. Author-unknown.
- ↑ Peer, Asaf. Killing Lone Cancer Cells Using Alpha-Particles. TFOT: The Future Of Things'. Web. September 28, 2007 .
- ↑ Nave, Rod. Radioactivity. Hyper Physics. Accessed November, 12, 2011.
- ↑ Three Types of Radioactive Decay. ORACLE ThinkQuest: Education Foundation.Accessed November, 12, 2011. Author-Unknown.
- ↑ physics.bu.edu. Elementary Physics II. Accessed November, 12, 2011.Author-unknown.
| ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||