Nuclear chemistry
Nuclear chemistry is a subfield of chemistry dealing with radioactivity, nuclear processes and nuclear properties. Of particular interest are the process involved with the splitting and combining of atoms to make new substances and energy.
History
Radioactivity was first discovered by accident in 1896 by a French scientist, Henri Becquerel. He was experimenting with fluorescent and phosphorescent materials to help understand the properties of x-rays and their ability to expose photographic film, which had been discovered in 1895 by Wilhelm Roentgen. Upon seeing x-ray exposed film, he immediately thought of putting some phosphorescent rocks on photographic paper to see if it would darken the film in the same way.[2] As Becquerel had anticipated, the phosphorescent salts produced an image on the film, but he was surprised to also find a strong and clear image when the rocks were not exposed to sun. During this fortuitous sequence of events Becquerel had discovered radioactivity.[3]
Marie Curie, who was one of Becquerel's students and her husband Pierre, continued to study radiation while working in Becquerel's lab. Henri Becquerel, Marie and Pierre Curie jointly received the Nobel Prize in physics in 1903 for their discovery of radioactivity and their other contributions in this area.[4]
Nuclear radiation
- Main Article: Radioactive decay

There are three main types of ionizing radiation known as alpha, beta, and gamma. Alpha particles are helium nuclei that have been emitted from a radioactive source. They have 2 protons and 2 neutrons and a 2+ charge. There are 3 types of Beta decay: electron emission, electron capture, and positron emission. During electron emission, a neutron changes into a proton with the loss of an electron. A gamma ray is a high-energy photon emitted by a radioisotope. Sometimes, nuclei emit gamma rays with alpha or beta particles during radioactive decay.[6]
| Property | Alpha radiation | Beta radiation | Gamma radiation |
|---|---|---|---|
| Composition | Alpha particle (helium nucleus) | Beta particle (electron) | High-energy electromagnetic radiation |
| Symbol | 42He or α | 0-1e or β | γ |
| Charge | 2+ | 1- | 0 |
| Mass | 4 | 1/1837 | 0 |
| Penetrating power | Low | Moderate | Very high |
Half-Life
A half-life is the time required for one-half of the nuclei of a radioisotope sample to decay to products. Some isotopes decay fast which means that they have a high specific capacity. On the other hand, others decay at a slower rate. Each isotopes have various range from a few microseconds to billion of years.
| Isotope | Half-life |
|---|---|
| Carbon-14 | 5730 years |
| Ismuth-212 | 60.5 seconds |
| Polonium-215 | 0.0018 seconds |
| Thorium-234 | 24.1 days |
| Sodium-24 | 15 hours |
| Uranium-238 | 4.5 billion years |
For example, you have 20 grams of Barium-139. It has a half-life of 86 minutes. After 86 minutes, you would have half of the sample, since the half of atom in sample would be decayed to another element which is Lanthanum-139. So after 86 minutes, which is a half-life of Barium-139, you have 10 grams of Barium-139 and 10 grams of Lanthanum139. After another 86 minutes, the half of 10 grams of Barium-139 would be decayed to Lanthanum-139. Therefore, you would have 5 grams of Barium-139 and 15 grams of Lanthanum-139. [1]
Some scientists use the half-life of carbon-14 to find the age of an object that was part of a living system. Most of Earth's carbon are carbon-12 and carbon-13 which are the stable form of carbon. Since high-energy cosmic rays from space keep producing carbon-14 in the upper atmosphere, the ratio of carbon-14 to other carbon isotopes is constant. All the living organisms live in carbon dioxide in the atmosphere. During their life, they keep exchange carbon with the environment. However, if an organism dies, it stop exchanging carbon with the environment. Also, its carbon-14 atoms decay without being replaced. So the ration of carbon-14 to other stable carbon in the remains of living organism could tell the archaeologist to find the approximate age of object.
Fission and Fusion of Atomic Nuclei
Nuclear Fission
- Main Article: Nuclear fission
An atom's nucleus can be split apart. When the nucleus is split apart, it releases lots of energy include heat and light energy. [2] A very small amount of the matter can release a very large energy. This energy could make electricity and if it is stronger and more powerful, it could be used as a weapon. For example, the fission of 1 kg of uranium-235 is same energy as the explosion of 20,000 tons of dynamite. Atomic bombs are examples of the uncontrolled nuclear chain reactions. To prevent this, fission should be controlled so energy is released slowly.
There is a useful device called nuclear reactors which use controlled fission to produce useful energy. The controlled fission reaction releases heat energy. In the core of the nuclear reactor, the heat energy is used to boil the water. So in the nuclear reactor, instead of burning the fuel, it uses chain reaction to split atom apart. The water from the core of the nuclear reactor is sent to another part. In the heat exchanger, it heats set of pipes which are filled with water and make them steam. And these steams turns a turbine to make electricity. [3]
Nuclear Fusion
- Main Article: Nuclear fusion
Another form of nuclear energy is when smaller nuclei combine together to produce a larger nucleus. This is called nuclear fusion. In solar fusion, the sun uses nuclear fusion of hydrogen nuclei to helium nucleus as you can see below equation. It releases the heat and light and other radiation. [4]
411H -> 42He + 20+1e + Energy
There is a very important difference between fusion reaction and fission reaction.
Fusion reaction -> small nuclei combine
Fission reaction -> large nuclei split apart
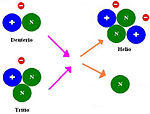
Compare to nuclear fission, fusion reactions release much more energy than fission reaction. But, fusion reaction occurs only at very high temperature like 40,000,000℃. Many scientists study for the use of controlled nuclear fusion to produce an energy source on Earth. One of the reaction that scientists are studying is the combination of a deuterium nucleus and a tritium nucleus. When a deuterium which is hydrogen-2 and a tritium which is hydrogen-3 combine together, they will form a helium nucleus. [5]
21H + 31H -> 42He + 10n + energy
 Browse |
References
- ↑ CNO cycle by Wikipedia
- ↑ Discovery of Radioactivity by Dr. V B Kamble. Vigyan Prasar Science Portal
- ↑ The Discovery of Radioactivity by the Lawrence Berkeley National Laboratory
- ↑ The Discovery of Radioactivity by Duke University Department of Chemistry.
- ↑ Why Are Some Atoms Radioactive? by the U.S. Environmental Protection Agency.
- ↑ Radioactive Decay The Bodner Group. Bodner Research Web.
- ↑ Antony Wilbraham, et. al. Chemistry. Upper Saddle River, New Jersey: Prentice Hall, 2008. p801
- ↑ Antony Wilbraham, et. al. Chemistry. Upper Saddle River, New Jersey: Prentice Hall, 2008. p805
- Alpha Radioactivity Unknown Author. HyperPhysics.
- Radioactive Half-Life Unknown Autohr. HyperPhysics.
- Nuclear Fission Multiple Authors. World Nuclear Association (WNA).
- Nuclear Fusion Jess Brewer. A Skeptic's guide.
- Nuclear Chemistry Unknown Author. Visionlearning.
See Also
| ||||||||||||||