Yttrium
| Yttrium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Y | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::39 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::88.9059 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 3, 5, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 5s2 4d1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 9, 2 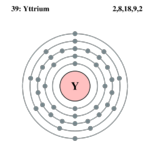
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-65-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density | [[Density::4.472 g·cm−3 g/ml]] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::1799 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::3609 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Yttrium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Yttrium is a chemical element which is part of the series of elements known as transition metals. Since its initial discovery in the late 1700s, yttrium has established itself as a useful and versatile element. Whether in the field of scientific research or through its commonplace function in household televisions, yttrium has become very important to modern life.
History
In 1787, a Swedish army lieutenant named Carl Axel Arrhenius found an interesting new stone near Ytterby, a small village near Stockholm. He gave the stone to Gadolin, a Finnish chemistry professor. Upon studying the rock, Gadolin discovered that a new element was in it; he named this element yttrium. In 1843, scientist Carl Gustaf Mosander discovered that the original stone was not pure yttrium, but that it actually contained a total of ten elements including yttrium. He named three of these erbium, terbium, and ytterbium after the town in which they were discovered. In this way, the town of Ytterby became the namesake for four new elements.[1][2]
Properties
Yttrium, a rare earth metal, has a silver-grey color and metallic luster. It also appears highly crystalline. Yttrium displays chemical properties similar to those of rare earth elements. It dissolves in both mineral acids and alkalis, which are chemical opposites of one another.[3]Yttrium is also slow to react with cold water. Yet when heated, yttrium rapidly responds and releases hydrogen gas.[4][5] Although yttrium is fairly stable in the air due to its protective oxide film, finely divided yttrium or powdered yttrium can become violently reactive when heated up to 400 degrees Celsius.[6][7][8]
Occurrences

Yttrium is a moderately abundant element found in Earth's crust. With its estimated abundance being 28 to 70 parts per million, yttrium is about as abundant as cobalt, copper, and zinc.[9] This element, which is often accompanied by scandium and other rare earth metals, is found in nature in the lanthanoids and most rare-earth minerals. Monazite, arguably the most important rare earth mineral, contains about 3% yttrium.[10][11] Yttrium has also been discovered in other parts of the solar system. For example, rocks from the moon contained very high amounts of it.[12][13][14] Xenotime, bastnasite, fergusonite, and smarskite ores have also been discovered to contain this element.[15][16] The primary mining areas for yttrium are the USA, Russia, Norway, and Madagascar.[17]
Extraction
The extraction of yttrium and lanthanoid metals from the ores is a complicated and lengthy process. To begin with, the metals are extracted from the ores using sulfuric acid, hydrochloric acid, and sodium hydroxide. By extracting the ores with these compounds, the ores are extracted as salts. After being extracted, the lanthanide salt mixture undergoes modern purification techniques such as complexation techniques, solvent extractions, and ion exchange chromatography. Another method of obtaining yttrium is through the reduction of the YF3 compound using calcium metal.[18] Four hundred tons of yttrium are produced annually and it is commercially available at approximately $75/oz.[19] However, it is usually traded in the form of yttrium oxide.[20]
Uses
Yttrium, a very versatile element, is utilized for a variety of purposes that affect everyday life. To begin with, yttrium is commonly used in laser systems, used in surgeries, weapons, and measuring tools. Yttrium oxide is probably one of the most useful compounds of yttrium and is responsible for its most common uses. This compound is combined with europium to produce phosphors that produce the red color in television tubes and computer screens. Hundreds of thousands of pounds of yttrium are consumed for this purpose alone. Other applications of yttrium oxide are in the production of microwave filters and the production of ceramic and glass formulas. Similarly, yttrium alloys have useful properties that are used in things like tools and jet engine coatings.[21][22]
Yttrium also has several uses in the scientific field. For example, the yttrium iron garnet is used as a transmitter of acoustic energy. In addition to this, small amounts of yttrium (0.1 to 0.2%) are used to reduce the grain size in the elements chromium, molybdenum, zirconium, and titanium. Those small amounts also strengthen aluminum and magnesium alloys. Yttrium can also be utilized as an additive to obtain alloys that have other useful properties. It also serves as a catalyst for ethylene polymerization reactions.[23]One isotope of yttrium, yttrium-90, is being used in the field of medical science. Because radiation given off by the isotope kills cancer cells, researches have hope that this easily-obtainable isotope will help cure cancer.[24]
Superconductivity
One of the most exciting properties of yttrium is its superconductivity. A superconductor is a material that has no resistance to the flow of electricity at a certain temperature. Superconductive materials could lead to major scientific breakthroughs and an increased efficiency of electricity. Technology today has machinery and wires that have some resistance to electrical energy, causing electrical energy to convert to heat. This means modern machinery is inefficient, because so much energy is lost. If superconductors like those containing yttrium can be used, there is the possibility for major advances in technology.[25][26]
Compounds or complex mixtures containing yttrium have been found to be superconductors. Scientists previously discovered materials displaying superconductivity when those materials were cooled to nearly absolute zero (about -273 degrees Celsius). These materials were difficult to work with due to the extremely cold temperatures. However, a recent discovery has made a major impact in this field of research. In January 1987, Paul C. W. Chu and a group from the University of Houston and the University of Alabama discovered superconductivity at 93K in a stable compound containing yttrium. YBa2Cu3O7, or YBCO, is superconductive at a temperature that is higher than that of liquid nitrogen. This is significant because scientists already know how to work with liquid nitrogen although its temperature is very cold. This means that the study of YBCO could not only lead to major breakthroughs in the study of superconductivity but also in the advancement of technology.[27][28]
References
- Yttrium Los Alamos National Labs. Chemistry Division.
- Yttrium Essential Information WebElements Periodic Table of the Elements.
- C&EN: It's Elemental: The Periodic Table - Yttrium Chemical and Engineering News.
- Yttrium (Y) - Chemical properties, Health and Environmental Effects Lenntech.
- Yttrium - Y Environmentalchemistry.com
- Yttrium Answers.com.
- Yttrium U.S. Geological Survey, Mineral Commodity Summaries, January 2003. Minerals.usgs.gov
- Yttrium-90 Therapy and 99 MTc pertechnetate knee uptake measurements in the management of rheumatoid arthritis. V Kyle, B L Hazleman, and E P Wraight. Pubmed Central Journal List.
- Y isotopes The Berkeley Laboratory Isotope Project.
- Yttrium Isotopes Chemical-elements.info.
- Element Yttrium Chemical-elements.info.
- Period Table of Elements: Yttrium
- Yttrium, Chemical ElementEnvironmentalchemistry.com.
- Yttrium David W. Brooks Teaching and Research Website.
| ||||||||||||||

