Ytterbium
| Ytterbium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Yb | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::70 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::173.054 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Lanthanide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | face-centered cubic, silvery white 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | N/A, 6, f | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14, 6s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2,8,18,32,8,2 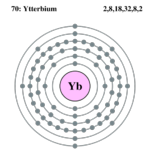
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-64-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density | Density::6.9 g/ml | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::824 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::1194 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Ytterbium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ytterbium is a chemical element that belongs to the lanthanide and known by the chemical symbol Yb and the atomic number 70. [1][2] Jean Charles Galissard de Marignac, a Swiss chemist, is credited for the discovery of this metal. It is assumed that the lanthanides do not generally occur in the earth; however, the name lanthanide, in fact, applies that these elements were extremely difficult to isolate from each other. Chemists manage to separate these metals so easily with the modern advanced techniques. Ytterbium has a bright silvery luster appearance. It is soft, malleable, and fairly ductile. [3]
Although this element is rather stable, it should be stored in sealed containers in order to prevent chemical reaction with water, oxygen, and mineral acids. Ytterbium has 38 total isotopes, of which only 7 are stable (Yb-174 being the most common). Ytterbium, while it usually has few known uses and poor commercial purposes, is usually used in a fiber laser operation machine and in a portable X-ray machine, and it is also used for giving strength to stainless steels and aluminum. Ytterbium is never discovered in nature as a free element (although can be found in mineral forms, such as mineral gadolinite, etc). [4] [1]
Creation and the beginning of chemistry - water
- Genesis 1:1-8. "In the beginning God created the heavens and the earth. Now the earth was formless and empty, darkness was over the surface of the deep, and the Spirit of God was hovering over the waters. And God said, 'Let there be light,' and there was light. God saw that the light was good, and he separated the light from the darkness. God called the light “day,” and the darkness he called 'night.' And there was evening, and there was morning—the first day. And God said, 'Let there be an expanse between the waters to separate water from water.' So God made the expanse and separated the water under the expanse from the water above it. And it was so. God called the expanse 'sky.' And there was evening, and there was morning—the second day.[5]
Properties
Physical properties of Ytterbium are those that result from the position and characteristics of its particles and that can be measured without causing a change in the identity of the material. [2]
Ytterbium is a face-centered cubic shaped and carries typical metallic conductive properties. This lanthanide metal is a bright, shiny surface, and is quite soft, malleable and ductile. Having two valence electrons, this element is fairly stable, but more reactive than other lanthanide elements. This landthanide is slowly reactive to water, oxygen, and can be easily dissolved by acids and ammonia, such as mineral acids and liquid ammonia.[3] Ytterbium has an atomic mass of 173.04 g/mol, density of 6.9 g/ml, melting point of 824 °C, and boiling point of 1194 °C.[3]
The Chemical properties of Ytterbium are those that describes how one substance reacts in the presence of other substances.[6] Of all the eight known ytterbium isotopes, one of them is the only stable form, and naturally occurring ytterbium is a mixture of seven stable isotopes. Ytterbium reacts slowly with water, oxygen, and can be easily dissolved by mineral acids; therefore, it should be kept in sealed container. This metal is stable at high pressures at room temperatures, but interestingly, when the pressure rises to 16,000 atm, it becomes a semiconductor in fiber optic cable, etc. The oxide, an anion of oxygen with oxidation number -2 builds a protective layer on the surface [3] [7]
Chemical Reactions
As described above in chemical property section, ytterbium slowly reacts with air. Chemically reacting, they form ytterbium(III) oxide, Yb2O3. The chemical equation is: [8]
4Yb + 3O2 → 2Yb2O3
- Chemical equation of ytterbium reacting with water. The metallic element is slowly reactive to cold water and quickly reactive to hot water to form ytterbium hydroxide, Yb(OH)3, and hydrogen gas (H2): [8]
2Yb(s) + 6H2O(g) → 2Yb(OH)3(aq) + 3H2(g)
- Ytterbium also reacts with halogens (a family VIIA element, which has seven valence electrons) to form ytterbium (III) halides: [8]
2Yb(s) + 3F2(g) → 2YbF3(s) [white]. When ytterbium reacts with fluorine (F2) to form ytterbium (III) fluoride, YbF3.
2Yb(s) + 3Cl2(g) → 2YbCl3(s) [white]. Ytterbium reacting with chlorine (Cl2) to form ytterbium (III) chloride, YbCl3.
2Yb(s) + 3Br2(g) → 2YbBr3(s) [white]. Ytterbium reacting with bromine (Br2) to form ytterbium (III) bromide, YbBr3.
2Yb(s) + 3I2(g) → 2YbI3(s) [white]. Ytterbium reacting with Iodine (I2) to form ytterbium (III) iodide), YbI3.
- Reaction of ytterbium with acids. Ytterbium slowly reacts to water and air, but easily gets dissolved by dilute sulphuric acid to form a colorless solution containing aquated (aq) Yb (III) with hydrogen gas (H2). This type of ytterbium reaction is not fully understood, but it is likely that the ytterbium ion [Yb(OH2)9]3+ is the largest form of Yb3+(aq) ion: [8]
2Yb(s) + 3H2SO4(aq) → 2Yb3+(aq) + 3SO42-(aq) + 3H2(g)
History
Ytterbium is a rare earth metal of the lanthanides with the atomic number 70, and the symbol Yb on the periodic table. The metal can be found in the gadolinite, monazite, xenotime, and bastnasite minerals. The Swiss chemist, Jean Charles Galissard de Marignac is technically considered to be the discoverer of this element. [4]
(Ce, La, Nd, Y)2 FeBe2Si2O10, the mineral gadolinite was discovered near the town of Ytterby, Sweden. The people used this mineral as the source of rare earth elements. In 1843, Carl Gustaf Mosander, a Swedish chemist, achieved to separate the mineral gadolinite into three components, and called them yttria, erbia, and terbia. Since Mosander named them so similarly and the three possessed analogous properties, the scientists soon became confused by erbia and terbia. By 1877, they had reversed their names, erbia as terbia and vice versa. [4]
A year later in 1878, a Swiss chemist, Jean Charles Galissard de Marignac, was able to separate erbia into two components. Marignac called the first component ytterbia, and the second the same, erbia. Eventually, Marignac postulated that ytterbia was actually a compound of a new element, which he called ytterbium. [4]
Soon after Marignac’s claim, other scientists produced ytterbium and experimented in order to record its properties. However, each of them all acquired different results from the same experiments, concluding that this was caused by their poor procedures and flaws. Suspicious of the abnormal empirical results, Georges Urbain, from France, considered that ytterbium was not an element, but a compound or a mixture of two elements. [4]
In 1907, Urbain succeeded in isolating ytterbium into two elements. As he had previously believed, ytterbium was in fact a mixture of two elements. Urbain named the first neoytterbium, meaning new ytterbium, and the other element lutecium. Sooner or later, the chemists changed the name neoytterbium back to its original name, ytterbium, and respelled lutecium to lutetium. [4]
Marignac, who was the first to separate the composition of ytterbia, is today ascribed for the discovery of the element ytterbium. Ytterbium lanthanide metal is a rare element today; however, it can be obtained through an ion exchange process from monazite mineral, (Ce, La, Th, Nd, Y)PO4, a substance full of rare earth elements. Monazite sand can be found in India, Brazil, Australia, and Africa. Ytterbium can also be obtained from bastnasite minerals from China and North America. The bastnasite mineral from Southern China is considered to have the highest concentration at 2-4 %.[9]
Occurrences
Ytterbium is one of the less abundant rare earths, but more common among the lanthanide metals. Scientific techniques, such as ion-exchange and solvent extraction techniques, built in the 20th century, have aided chemists with separating ytterbium from minerals. It makes 2.7 to 8 mg/kg in the Earth’s crust, which indicates that this metal is to some extent less abundant than boron, molybdenum, and thallium, but slightly more common than bromine, uranium, tin, and arsenic. The monazite mineral, a complex phosphate that can be found in Brazil, India, mainly Florida, and other various places, is the most common and important form of ytterbium sources.[10]
The other ytterbium ores are euxenite, gadolinite, and xenotime. Euxenite, mainly found in Idaho, United States, is a brownish black mineral with a metallic luster that also contains thorium and uranium elements. Gadolinite, mainly occurring in Scandinavia, includes beryllium and iron silicate. Lastly, xenotime, a phosphate of ytterium in crystalline form, is a brownish yellow mineral that can be discovered in pegmatites, igneous rocks, and so on. Xenotime also occurs with monazite minerals. [9]
Uses
Ytterbium has few known uses. A small amount of the metal can be abstracted to add strength and grain refinement to mechanical properties of stainless steel and aluminum, and further functions as a semiconductor in fiber optic cable. One isotope of ytterbium (Yb-169) has been used as a radiation source substitution for a portable X-ray machine when electricity is unavailable. The isotope is also used in gamma cameras for radiation sources, and also for the medical treatments for prostate cancer, such as I-125, and Pd-103. Chemists use Yb-176 isotope for the production of a lutetium isotope, Lu-177. Furthermore, Yb-171 isotope, when excited, can be applied in a laser device.[4]
Ytterbium is inserted in limited phosphor applications as well as in various types of specialized catalysts. Additionally, some ytterbium metal can be utilized in the production of a laser device, a device that produces very bright light of a single color and cuts through impenetrable objects, such as aluminum and metals, etc. This lanthanide metal, however, has poor commercial uses due to its annual production of 50 tonnes (a unit of weight equivalent to 1000 kilograms) per year.[11] [10]
Isotopes
There are 38 known ytterbium isotopes. Isotopes are ones that have atoms with the same number of protons (atomic number) but with different numbers of neutrons, resulting in different numbers of atomic mass. Seven stable ytterbium isotopes: Yb-168, Yb-170, Yb-171, Yb-172, Yb-173, Yb-174, and Yb-176 make up naturally occurring ytterbium. Yb-168 is used for the production of Yb-169, which is a radioisotope that is applied in gamma cameras for radiation sources. Yb-169 is also used for the prostate cancer treatments, such as I-125, and Pd-103. Yb-176 is used for the production of a lutetium isotope, Lu-177. Excited Yb-171 isotope can be utilized in a laser device, while the other ytterbium isotopes are applied in various physics experiments. Yb-174 is the most common among the 38. [12]
38 Yb isotopes (stables are indicated): [12]
| Name of the Isotope | Atomic Mass (g/mol) | Half-Life |
|---|---|---|
| Yb-148 | 147.96742 | ~250 ms |
| Yb-149 | 148.96404 | 0.7 seconds |
| Yb-150 | 149.95842 | ~700 ms |
| Yb-151 | 150.95540 | 1.6 seconds |
| Yb-151ml | N/A | 1.6 seconds |
| Yb-152 | 151.95029 | 3.04 seconds |
| Yb-153 | 152.94948 | 4.2 seconds |
| Yb-154 | 153.946394 | 0.409 seconds |
| Yb-155 | 154.945782 | 1.793 seconds |
| Yb-156 | 155.942818 | 26.1 seconds |
| Yb-157 | 156.942628 | 38.6 seconds |
| Yb-158 | 157.939866 | 1.49 minutes |
| Yb-159 | 158.94005 | 1.67 minutes |
| Yb-160 | 159.937552 | 4.8 minutes |
| Yb-161 | 160.937902 | 4.2 minutes |
| Yb-162 | 161.935768 | 18.87 minutes |
| Yb-163 | 162.936334 | 11.05 minutes |
| Yb-164 | 163.934489 | 75.8 minutes |
| Yb-165 | 164.93528 | 9.9 minutes |
| Yb-166 | 165.933882 | 56.7 hours |
| Yb-167 | 166.934950 | 17.5 minutes |
| Yb-168 | 167.933897 | Stable |
| Yb-169 (radioactive) | 168.935190 | 32.026 days |
| Yb-169m | N/A | 46 seconds |
| Yb-170 | 169.9347618 | Stable |
| Yb-171 | 170.9363258 | Stable |
| Yb-172 | 171.9363815 | Stable |
| Yb-173 | 172.9382108 | Stable |
| Yb-174 | 173.9388621 | Stable |
| Yb-175 | 174.9412765 | 4.185 days |
| Yb-176 | 175.9425717 | Stable |
| Yb-176m | 11.4 seconds | |
| Yb-177 | 176.9452608 | 1.911 hours |
| Yb-177m | N/A | 6.41 seconds |
| Yb-178 | 177.946647 | 74 minutes |
| Yb-179 | 178.95017 | 8.0 minutes |
| Yb-180 | 179.95233 | 2.4 minutes |
| Yb-181 | 180.95615 | ~1 minutes |
Compounds
There are 13 known Ytterbium compounds - eleven diatomic compounds and two polyatomic compounds: [13]
Diatomic Compound are the chemical compounds that consist of more than two atoms of different elements chemically bonded together. [6] Diatomic compounds contain only two different elements. Ytterbium compounds are rare on the earth; however, there are 14 known ytterbium compounds:
- YbF2 - Ytterbium difluoride - This compound appears grey or grey-green, exhibits crystalline solid shape, and having a melting point of 1407 C and a boiling point of 2380 C. Ytterbium difluoride consists of 18.00 percent fluorine and 82.00 percent ytterbium with oxidation number +2. It has formula weight of 211.037 g/mol.
- YbF3 - Ytterbium trifluoride - possesses a mass of 230.035 g/mol. This compound is white and shaped as crystalline solid. Ytterbium trifluoride includes melting point of 1157 C and boiling point of 2230 C. Its density is 8200 kg m-3. Fluorine consists of 24.78 percent while ytterbium has 75.22 percent of element. [15]
- YbCl2 - Ytterbium dichloride - carries a weight 243.945 g/mol. This compounds appears as green or yellow-green, having crystalline solid shape. Melting point and boiling point of this compound are 720 C and 1900 C, and has density of 5270 kg m-3. Element percentile is 29.07 Cl, and 70.93 Yb. [16]
- YbCl3 - Ytterbium trichloride - weighs 279.398 g/mol. The color of this compound is white, and appears as crystalline solid, having melting point of 865 C. Ytterbium trichloride compromises of 38.07 Cl and 61.93 Yb. [17]
- YbBr3 - Ytterbium tribromide - This compound is white, and exhibits crystalline solid appearance. The melting point of ytterbium tribromide is 959 C, and weights 412.752 g/mol. bromine elements. [18]
- YbI2 - Ytterbium diiodide - This compound is yellow, resembles crystalline solid structure, and has the melting point of 780 C and formula weight of 426.849 g/mol. Ytterbium diiodide compromises of 59.46 percent iodine and 40.54 percent ytterbium. [19]
- YbI3 - Ytterbium triiodide - is a white or yellow colored compound that exhibits crystalline solid appearance. It has the melting point of 700 C, and weights 553.753 g/mol. Iodine consists of 68.75 percent while ytterbium composes of 31.25 percent. [20]
- Yb2O3 - Diytterbium trioxide - composes 394.078 g/mol. It is white and appears as crystalline solid. This compound has a high melting point of 2435 C, and density of 9200 kg m-3. Diytterbium trioxide composes of 12.18 percent oxygen and 87.82 ytterbium. [21]
- Yb2S3 - Diytterbium trisulphide - the color, melting and boiling point, and density of this compound have not yet been recorded. Its formula weight is 442.278 g/mol. Diytterbium trisulphide contains 21.75 percent sulfur and 78.25 ytterbium. [22]
- YbSe - Ytterbium selenide - This compound appears as solid; however, the color, melting and boiling point, and density have not been recorded. It weighs 252 g/mol. Ytterbium selenide carries 31.33 percent selenium and 68.67 percent ytterbium. [23]
- Yb2Se3 - Diytterbium triselenide - has appearance of solid. The color, melting and boiling point, and density are yet to be recorded. Its formula weight is 582.96 g/mol. Percent composition of this compound is 40.63 selenium and 59.37 ytterbium. [24]
- YbTe - Ytterbium telluride - has a percent composition of 42.44 tellurium and 57.56 ytterbium. This compounds weighs 300.64 g/mol and appears as solid. [25]
Polyatomic Ytterbium Compounds
Polyatomic compound are those that contain at least three different elements (Examples: NaNO3 and NH4OH)[2]
There are two polyatomic ytterbium compounds:
- YbCl3·6H2O - Ytterbium trichloride hexahydrate - This complex compound has formula weight of 387.49 g/mol. Its color is green, exhibits crystalline solid, and has the melting point of 150 C and the density of 2570 kg m-3. The percent composition of ytterbium trichloride hexahydrate is 27.45 percent chlorine, 3.12 hydrogen, 24.77 oxygen, and 44.66 ytterbium. [26]
- Yb2(SO4)3·8H2O - Diytterbium trisulphate octahydrate - This another complex compound type of ytterbium weighs 778.393 g/mol. It is white and exhibits crystalline solid appearance. The density of this complex compound is 3300 kg m-3. Diytterbium trisulphate octahydrate compromises of 2.07 percent hydrogen, 41.11 oxygen, 12.36 sulfur, and 44.46 percent ytterbium. [27]
Ytterbium Fiber Laser
Ytterbium is perhaps the most known for fiber laser cutting operation. Fiber lasers are often used in the medical field, spectroscopy, and in materials processing, etc. Fiber lasers need rare earth elements for proper function, rare earth element such as ytterbium. They are used in cutting metals, nonmetals, and organics (some are familiar with cutting of aluminum). The wavelength, power, beam quality, and spot size, etc, determine the efficiency of the laser operation. [28]
The most efficient wavelength is at 1070 nm (nanometer) for the operation. Fiber lasers have wide power range, the beam focus, and its position remain constant; therefore, even in a case when the laser power is shifted, the processing results are most likely to remain consistent. Adjustment of the optics configuration allows the machine to have a wider range of spot sizes. Power density is also adjustable for different types of materials and their thickness, from metal to nonmetal, etc. Better beam quality and small spot size of the machine help in cutting thin materials that will eventually be used in micromachining applications. High power density with small spot sizes of the laser result in better cutting edge quality. In addition, the large depth of field and small spot sizes possibly lead to straight walls in thick metals. [28]
Examples of the fiber lasers are micromachining cardiovascular stents, resize silicon wafers for solar panels, 3-D cutting, high pressure clean cutting of stainless steels and aluminum, tube cutting, 2-D cutting, etc. Mild steels, stainless steels, titanium alloys, aluminum alloys, galvanized steels, and plastics are among the most common materials that the fiber lasers cut.[28]
Abundance
Ytterbium is one of the rare earth lanthanide metals found mostly in minerals. However, this particular element was analyzed to be present in the universe, sun, meteorite (carbonaceous), crustal rocks, sea water, stream, but no data in human. In this chart, ppb is parts per billion; 1 billion is 109 for weight and numbers of atoms. [29]
| Location | weight (ppb) | atoms (ppb) |
|---|---|---|
| Universe | 2 | 0.01 |
| Sun | 1 | 0.01 |
| Meteorite (carbonaceous) | 180 | 20 |
| Crustal rocks | 2800 | 340 |
| Sea water | 0.0008 | 0.000029 |
| Stream | 0.05 | 0.0003 |
| Human | N/A | N/A |
Video
Ytterbium general information [30]
Fiber laser cutting 10th inch aluminum [31]
Gallery
References
- ↑ 1.0 1.1 Facts about Ytterbium Facts about Index, 2 Dec. 2010.
- ↑ 2.0 2.1 2.2 Cox, Porch, and Wetzel. Chemistry for Christian Schools. South Carolina: Bob Jones University Press, 2000. (p.537).
- ↑ 3.0 3.1 3.2 3.3 Lenntech Ytterbium - Yb Lenntech Water treatment & purification Holding B.V, 2 Dec. 2010.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Ytterbium Jefferson Lab, 20 Nov. 2010.
- ↑ Genesis 1 Biblegateway.com, 2 Dec. 2010.
- ↑ 6.0 6.1 Cox, p.530
- ↑ Periodic Table of Elements Element Ytterbium-Yb Kenneth L Barbalace, EnvironmentalChemistry.com, 2 Dec. 2010.
- ↑ 8.0 8.1 8.2 8.3 8.4 Chemical reactions of ytterbium Mark Winter, The University of Sheffield and WebElements Ltd, UK, 2 Dec. 2010.
- ↑ 9.0 9.1 Occurrences Ytterbium, 2 Dec. 2010.
- ↑ 10.0 10.1 Ytterbium Advameg, Inc, 2 Dec. 2010.
- ↑ ytterbium University of California for the US Department of Energy, 2 Dec. 2010.
- ↑ 12.0 12.1 12.2 Ytterbium isotopes Ytterbium, 2 Dec. 2010.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 2 Dec. 2010.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ http://www.webelements.com/compounds/ytterbium/ytterbium_tribromide.html Ytterbium compounds] Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ Ytterbium compounds Mark Winter, The University of Sheffield and WebElements Ltd, UK, 11 Jan. 2011.
- ↑ 28.0 28.1 28.2 Laser Cutting with Ytterbium Lasers IPG Photonics, 2 Dec. 2010.
- ↑ 29.0 29.1 Abundance Mark Winter, The University of Sheffield and WebElements Ltd, UK, 2 Dec. 2010.
- ↑ Ytterbium Periodic Table of Videos periodicvideos, youtube.com, 19 Jan, 2009.
- ↑ Fiber Laser Cutting 10th inch Aluminum LaserPhotonics, Youtube.com,16 Jan. 2009.
Additional Information
- Ytterbium images A Visual Museum, 2 Dec. 2010.
- Ytterbium lists of videos Youtube.com, 2 Dec. 2010.
- Ytterbium periodic table Eni Generalic, KTF-SPLIT EniG., 4 Jan. 2003.
- Ytterbium more data sheet ChemGlobe.org, 2 Dec. 2010.
- Ytterbium iscid Jason Evan Stalnaker, International Society for complexity, Information, and Design, 2 Dec. 2010.
- Chemistry Explained ChemistryExplained.com, 2 Dec. 2010.</ref>
- Ytterbium basic information EnvironmentalChemistry.com, 2 Dec. 2010.
- Periodic Table:Ytterbium Yinon Bentor, Yinon Bentor, 2 Dec. 2010.
- ytterbium information University of California for the US Department of Energy, 2 Dec. 2010.
- Ytterbium wikipedia Wikipedia.com, 2 Dec. 2010.
- Ytterbium analysis Mark Winter, The University of Sheffield and WebElements Ltd, UK, 2 Dec. 2010.
- Fiber laser pictures Flicker.com, 2 Dec. 2010.
- Ytterbium facts Ytterbium, 2 Dec. 2010.
- Ytterbium’s Broken Symmetry A U.S. Department of Energy National Laboratory Operated by the University of California, 2 Dec. 2010.
| ||||||||||||||












