Sodium
| Sodium | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
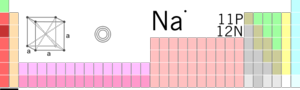
| |||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Na | ||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::11 | ||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::22.98976928 g/mol | ||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Alkaline earth metals | ||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white 
| ||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 1, 3, s | ||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | 3s1 | ||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2,8,1 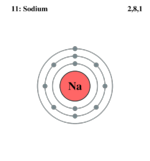
| ||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-23-5 | ||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||
| Density | Density::0.968 g/ml | ||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::370.87 K | ||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::1156 K | ||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Sodium | |||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||
Sodium is a chemical element typically abbreviated with the symbol Na. It is found in numerous naturally occurring compounds including the mineral halite. It is very soft and smooth with a silvery metallic color that burns with a yellow flame. It is considered to be one of the alkali metals and is very reactive - even with water - requiring storage in an inert environment.
History of Sodium

Sodium Chloride and Sodium Carbonate have been known of since the time of antiquity. It was mostly Egyptians who first used sodium carbonate. They not only used it to eat as a source of food and sustenance, but also as a way of preserving their mummies as well. Evidences of this include Egyptian art containing references to salt as long ago as 1450 B.C. [1] Sodium would occur naturally, in a large part of the ashes of marine algae. Sodium Hydroxide was originally discovered by a man named sir Humphrey Davy.[2] When Mr. Davy discovered it, he was in England in the year 1807. At the time that sodium hydroxide was discovered, it was considered an elementary substance and was called a fixes alkali. All the way up until Mr. Davy, there had been no real difference to anyone separating sodium and potassium. Scientists in the older days did not understand that the “Vegetable Alkali” was K2C03, and the “mineral alkali” was Na2CO3. Eventually scientists realized that the two elements were not the same, and the distinction was made between the two elements. Mr. Davy made the element through the process of electrolysis with very dry molten sodium Hydroxide, NaOH. Sodium was found collected at the cathode. A very similar procedure was used by Mr. Davy to isolate potassium. The name sodium was derived from the English word “soda.” The reason that sodium was given the letters Na, is because it comes from a Latin word “Natrium.” [3]
Properties

Sodium is classified as a soft metal. It is a soft, bright, silvery metal which could float on the surface of water if it did not oxidize so violently in water. [4] Its atomic number is 11 with the symbol “Na”. It weighs approximately 22.9898 gm/mole.[5] It has a low melting point and has an approximate density of .97 at 20 degrees Celsius (68 degrees Fahrenheit). It is commonly used, and therefore considered to be very important. Because of the properties that is has, such as reactivity with other elements, it is commonly used as an ingredient in many household cleaners, such as washing soda. This is not the only practical use of sodium, because of its properties that cause it to expand when introduced to many mixtures, it is commonly used in cooking in the form of baking powder, to help with raising the dough of the item being cooked. Water, snow, and ice will react quickly and violently with sodium. When they react, they leave behind traces of sodium hydroxide and hydrogen. When freshly cut metallic sodium is left exposed to the air, it will turn from its original silvery color to a more opaque grey color. This is due to the sodium oxide coating formation around it. Even though sodium reacts violently with water, and reacts with other elements such as ammonia, there are some things with which it does not react at all. Sodium will not react with nitrogen. Hydrogen will react with sodium at temperatures above 200 degrees Celsius (390 degrees F) to create sodium hydride. Another element that sodium will react with is carbon, but the reaction is very minor. Halogens will often react to sodium, for example, sodium chloride is formed when sodium reacts with a few various halides. Alcohol has the same effect on sodium as water; however the reaction is a bit slower.[6]
Occurrences
Sodium is commonly found in stars. It is the most abundant alkali metal in our world's crust, taking up about ¼ of the total crust. Sodium fluoroacetate can easily be found in many plants across Brazil, Africa, and Australia. It was established as the poisonous substance of the leaves of Dichapentalum cymosum. This is a form of poison used to kill rats. Tea leaves are also believed to contain some trace amounts of sodium fluoroacetate. The Gastrolobium, a type of Australian plant also has the compound in the tips of the leaves and seeds. [7]. Sodium used to be chemically separated by heating sodium carbonate with carbon at 1100 degrees Celsius. This was less effective, and less productive than the alternative that is used today. Now, it is created through the electrolysis of liquid sodium chloride.[8] Sodium Chloride is found in enormous quantities in ocean water. Great halite deposits carry an equal amount of NaCl2. They were formed by prehistoric seas being evaporated or in salt lake beds that have dried. The USA, United Kingdom, France, Germany, China, and India contain such deposits. There are many ores that also carry sodium. Borax, the caryollite and chile nitrate also carry sodium.[9] Even though sodium can be found in many places in many combinations with other elements, it is rarely seen unattached to another element. Because of its highly reactive properties, due to its single valence electron, it is extremely reactive with chlorine, which has seven valence electrons, forming NaCl. This is table salt.
Uses
Manufacturing Benefits
There a number of uses for sodium. When combined with chlorine, it becomes a substance by the name of NaCl, or better known as table salt. NaCl is one of the most important compounds found anywhere in the living environment. Because of its metallic properties, sodium is used in making esters and also in manufacturing of organic compounds. Sodium hydroxide is commonly used in the productiion of paper, soap, rayon, textiles, and some forms of rubber. Borax is made of the simple element of sodium tetraborate. In toothpaste that everyone uses most days, sodium fluoride is nearly always used with sodium acting as the cleaning agent for your teeth. Rat poisons and ceramics also contain sodium fluoride. Sodium Carbonate is also required to create the many different forms of glass that are used every day. [10] Some forms of sodium are used in fertilizer. [11]. Sodium is also used in nearly all dietary substances. [12] Sodium is also used in agriculture as well as in medicines. Sodium nitrate, a common meat preservative, can be solely implemented in medicine to treat cyanide poisoning. It holds potential of someday being used to protect and keep the tissue and organ functions after trauma to the organs is sustained. [13]Sodium is also widely used in manufacturing businesses because of its strengthening properties. Many businesses will use sodium to improve the structure of many different alloys. it hes previously been used in soap, many fatty acids, to decal metals, purify molten metals, and in lamps that illuminate with sodium vapor.
Cleaning and Cooking Properties
Sodium carbonate is also better known as washing soda which can be commonly used to cut grease, clean petroleum oil, remove wax or lipstick, and neutralize odors with the same power as baking soda does. Washing soda is actually the same as baking soda, other than it has been processed differently. It has the power of a cleaning agent with a pH of 11, only it doesn't emit fumes that are harmful to humans. Many people use washing soda for everyday use in their homes. The baking soda that many recipes call for is also made of sodium bicarbonate. Because of its ability to expand, baking soda is used commonly in cooking. Baking soda in any cake, muffin, scone, etc... causes the dough to rise. Baking soda is in detergents, sometimes sodium silicates are used as fillers.
Isotopes
There are up to thirteen known sodium isotopes, although only one of the known isotopes is stable, 23Na. Two of the isotopes, 22Na, and 24Na are radioactive. Sodium-22 has a half life of about two and a half years. Sodium-24 has a half life of about fifteen hours. Sodium-23, a safe isotope found in blood plasma can be converted into 24Na via neutron radiation. Based on the amount of the 24Na isotope that can be found in the blood, it is possible to accurately determine how much neutron radiation dose was given. [14] Sodium-22 emits a positron of 546 keV and a gamma ray of 1,274.5 keV. It has an activity concentration of >10 mCi/ml, and a radiopurity of >99.9%. [15]
Gallery of compounds
Sodium phosphate crystals
Sodium chloride (ordinary table salt)
Sodium bicarbonate (baking soda)
Sodium fluoroacetate in trace amounts in the tips of the leaves and seeds of the Gastrolobium.
References
- Sodium Wikipedia
- principal metals principalmetals.com
- properties of sodium lenntech.com
- occurrances of sodium carycademy.org
- uses of sodium essay depot
- occurance of sodlum supplement news
- medical uses for sodium national institutes of health
- the history of sodium history of sodium
- the history of salt history of salt
| ||||||||||||||








