History of the Periodic Table
The history of the periodic table involves over a century of growth in the understanding of chemical properties. Chemists have always looked for ways of arranging the elements to reflect the similarities between their properties. The modern periodic table lists the elements in order of increasing atomic number (the number of protons in the nucleus of an atom). The most important event in its history occurred in 1869, when the table was published by Dmitri Mendeleev, who built upon earlier discoveries by scientists such as Antoine-Laurent de Lavoisier and John Newlands, but who is nevertheless generally given sole credit for its development.
However, before the creation of the periodic table, the elements first had to be discovered. After the discovery of pure substances, chemists began to make a list of elements. This list grew until it had over sixty elements. Scientists began to see patterns in elements, like color and density. John Newlands had the idea to arrange them by atomic weight, and it was Dmitri Mendeleev who accomplished this task. After that, the periodic table continued to grow, and the rules about it changed as elements were added and rearranged. Today the periodic table is an organized representation of all the elements we have discovered. Not many changes are being made anymore, but it continues to aid scientists in other discoveries and accomplishments.
Early Organizational Endeavors
In the Middle Ages, alchemists realized that some of the substances they were working with were pure. Meaning that they were not composed of other substances. In 1793, the first list of elements was published. It was Antoine Lavoisier, a great French chemist, that created a list of thirty substances that would not break down, and named them elements. Later in 1803 John Dalton came up with his own list of elements. He revised his list several times, and eventually ended up with sixty documented elements. [1]
The Discovery of Element Periodicity
In 1869, 63 elements had been discovered. As the number of elements grew larger, scientists begin to see certain patterns in the elements. [2] In 1829, Johann Dobereiner observed that there were groups of elements with properties that were similar. Chlorine, bromine, and iodine were some of the elements that he saw similarities in. These elements were gases, had a similar color, and other similar qualities. Dobereiner begin to construct triads with the elements, but these eventually fell through and did not all fit together. [1] Then in 1864 John Newlands thought that the elements may be able to be arranged according to atomic weight, so he tried to arrange them in order of increasing atomic weight. This idea would not work because it groups elements together that have different chemical properties. But, he did take steps in leading to the creation of the periodic table. Newlands was the first person to add atomic numbers to the elements, which are on the periodic table to this day. [3]
Mendeleev's Periodic Table
Almost all of the credit for the periodic table goes to Dmitri Mendeleev. He arranged the elements by their atomic mass, like Newlands, but he did something different. He added in the elements that had not been discovered yet. He decided to do this because while he was studying the atomic masses he noticed that when they were put in order some of them increased more than others. [1] He also arranged them vertically [4], and added the transition metals. He recognized that the transition metals did not fit into the chart so he separated them from the rest of the periodic table. Mendeleev summarized his work by writing the periodic law. The law states, The properties of elements vary with their atomic masses in a periodic way. [1]
The Modern Periodic Law
Although Mendeleev's periodic table was brilliant, there were still some things wrong about it. The way that the elements were arranged still did not always group the elements that were similar. One of Ernst Rutherford's assistants, Henry Moseley, suggested that they develop a way to count the number of protons in an atom's nucleus. Once he created a way to do this, he realized they needed to revise the periodic table to make it more accurate, now that they could find the number of protons in an atom. He changed the periodic table and the periodic law. The modern periodic law states, The properties of elements vary with their atomic numbers in a periodic way. [1]
Artificial Elements
The only large addition to the Periodic Table was after World War II. The experiments with nuclear energy created heavy elements. These elements created in man-made nuclear reactions were called transuranium elements. They were given this name because their atomic numbers are greater than uranium's atomic number. Neptunium and plutonium are the only transuranium elements found in nature. The rest of them are products of the nuclear reactions. [1]
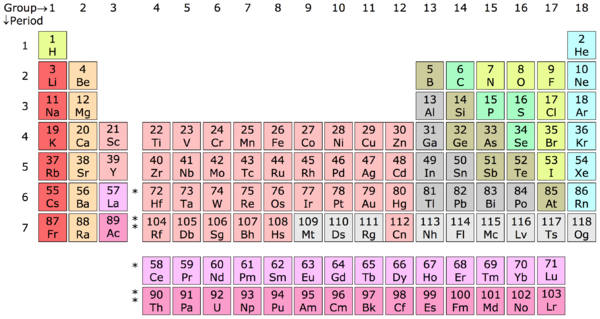
The Modern Periodic Table
The modern periodic table has more than the 63 elements that were discovered by Mendeleev. Most of the periodic tables up to this date have up to 109 elements. It has 18 groups in vertical columns. The vertical columns are labeled by roman numerals or numbers, and each horizontal row is called a period, and the periodic table consists of seven periods. [5] On the left and middle side of the table there are the elements that are metals. On the right side are the nonmetals, groups 13-18. The metalloids are adjacent to the stair-step line on the table. On the bottom of the table are the tow rows of elements called the lanthanide series and the actinide series. The atomic number increases from left to right along the whole periodic table, and become less metallic.
Element Families
There are distinct families in the periodic table. One example is the alkali metal family. It consists of all the elements in the periodic table that are very chemically reactive. The elements are lithium, sodium, potassium, rubidium, cesium, and francium. These metals conduct electricity easily and have a bright and shiny surface. They all react easily with water. Alkali metals are most commonly used in streetlights, soap, rayon, and paper. There are many different families. The others are: alkaline-earth metals, transition metals, post-transition metals, metalloids, nonmetals, halogens, and noble gases. [1]
Facts About the Periodic Table
- There are ninety elements in the periodic table that are found in nature, the rest are man-made.
- Most of the elements in the periodic table are metals.
- The main difference between Mendeleev's periodic table and the modern periodic table is that Mendeleev's table has the elements arranged by increasing atomic weight, and the modern periodic table has the elements arranged by increasing atomic number.
- The first element to be made artificially was Technetium. [6]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Batdorf, Brad. [The Periodic Table, Chapter 5] BJU Chemistry, Third Edition. Published 2009.
- ↑ Western Oregon University. A BRIEF HISTORY OF THE DEVELOPMENT OF PERIODIC TABLE wou. Accessed May 21, 2017
- ↑ Purdue. John Newlands Purdue edu. Accessed May 21, 2017.
- ↑ BBC. Atoms and the periodic table BBC Science. Accessed May 21, 2017.
- ↑ GENESIS. Cosmic Chemistry: An Elemental Question Genesis Education. Accessed May 21, 2017.
- ↑ ThoughtCo. 10 Periodic Table Facts ThoughtCo. Accessed May 21, 2017.
| ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||