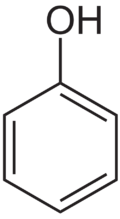
Phenol
| Phenol | |
|---|---|
| |
| General | |
| Systematic name | Phenol |
| Other names | Monohydroxybenzene, Benzenol,
Phenyl hyroxide, Phenylic acid |
| Molecular formula | C6H5OH |
| Molar mass | Molar mass::94.11 g/mol |
| Appearance | Colorless to light pink crystalline solid |
| CAS number | CAS number::108-95-2[1] |
| Properties | |
| Density and phase | Density::107 g/ml, ? |
| Solubility in water | 8.3 g/100 ml (20°C) |
| Melting point | Melting point::42°C |
| Boiling point | Boiling point::182°C |
| Acidity (pKa) | 9.95 |
| Basicity (pKb) | 1.3 x 10 mol dm-3[2] |
| Structure | |
| Molecular shape | Planar |
| Dipole moment | ? D |
| Hazards | |
| MSDS | Material safety data sheet |
| Main hazards | Toxic and harmful to human |
| NFPA 704 | |
| Flash point | 79°C |
| RTECS number | SJ3325000 |
| Related compounds | |
| Related compounds | Benzenethiol[1] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Phenol is a white crystalline organic compound with the chemical formula C6H5OH. It is said that the taste of phenol is a burning sensation and has a pungent aroma. Phenol is perhaps best known for its widespread industrial use in the making aromas, plastic, flavoring, drugs like aspirin, and also adhesives.[3] Phenol is uncommon on its own, yet, like how God made people, when phenol is combined with other compounds it becomes greater together. Such as God says in the Bible that we sharpen each other, so does other compounds with phenols.
Properties
Phenol is an organic compound that has a white crystalline mass that can change colors to red or pink depending on the air that it is exposed to.[4] The crystals are sometimes wet when they are discovered. When being handled, great care is needed because if the phenol contacts the handler's skin immediate white blistering will appear.Cite error: Invalid <ref> tag; invalid names, e.g. too many It may harm your skin because phenol is an acid. That acidic part of the compound was once formally called carbolic acid, but only when phenol was used as an antiseptic. [5] Phenol is also soluble, able to be dissolved, in water, at a rate of 84.2 g dissolving in 1000 mL. [6]
Natural Occurrences
Phenols are most commonly found in nature. Phenols in nature include tyrosine, an important standard in amino acids mostly found in proteins. Epinephrine, more commonly known as adrenaline is also a stimulant hormone made by the adrenal medulla. Serotonin, which is a neurotransmitter, chemical substance that is released by the arrival of a nerve impulse, in the brain. Urushiol, which is what is secreted by poison ivy, [7] and also the temporal gland, which is a secretion by the male elephants. [6] While many of the other more intricate compounds of phenols are used in flavoring and aromas. Of the many examples of that, one of the most familiar flavorings would be vanilla. There are a plethora of phenols that are obtained by plants other than vanilla such as thymol ,which is isolated from the spice thyme, and eugenol, which is isolated from the spice of cloves.[7]
Uses
Phenol is more commonly used than some people would think. One of its important uses would be that phenol is used to prepare reagents, substance or mixture for use in chemical analysis or other reactions, in plastic. [8] Plastic may not seem so important but it is essential in our day to day lives. Plastic is used in some of our shelters, clothing, transportation, entertainment, and also in a person's health care. [9] All this is possible because phenol plays a part in making plastic. This happens by the condensation reaction from the phenol by the acetone, that the phenol produced, produces bisphenol, which is also used extensively in the polymer industries. [8] It is also used in making many pharmaceutical drugs, such as aspirin, and herbicides.[6]
And while phenol is a toxic compound, if used in small portions it can be helpful around your house. Phenol is used in household cleaners and also in mouthwashes. Phenol is also another component in wood preservatives like creosote. Other substituted phenols are used in the making of dyes and also intensely colored azo dyes. Another component of phenol is used to help develop photographs. [7] Azo dyes fall into special categories depending on their own chemical features, which contain phenol. Azo dyes are not used to color food, they are used for dyeing cotton.[10]
History
In 1834 phenol became know by a German chemist, Friedlieb Ferdinand Runge. Runge extracted phenol from coal tar, but in an impure form. Runge then originally called phenol Karbolsäure, which means carbolic acid. However phenol in its pure form was obtained from a french chemist, Auguste Laurent, in 1841. Although before Laurent found phenol in its pure form he had coined the term "phène" for benzene. This would later become the base component of the word phenol. The compound had finally received the name phenol by another French chemist by the name of Charles Gerhardt, in 1843. Then Sir Joseph Lister had used phenol in his antiseptic surgery. Lister had put a piece of rag on what he thought were thoroughly cleaned wounds. The patient's skin became irritated because of the exposure to phenol which eventually led to the germ-free techniques in surgeries.
The toxicity of phenol can seriously effect on the central nervous system. Phenol causes both humans and animals to suddenly collapse and loss consciousness. Phenol was originally used by the Nazi's in the late 1930's. Although the Nazi's had used other chemicals in their gas chambers, phenol was also used to kill people by injection. They were given to hundreds of people, mostly in Auschwitz. It is said that approximately one gram of phenol could cause the death of a single person.[6] Johann Paul Kremer, a doctor who was a SS (Schutzstaffel), had arrived in Auschwitz. Kremer was there to take the place of a sick doctor. Kremer ordered most of the Jews to be killed by an injection of phenol. Kremer and the other Nazi doctors kept very detailed autopsy reports of the effects of the phenol injections.[11]
Video
The process of making phenol.
References
- ↑ 1.0 1.1 Phenol Research New World Encyclopedia. Web. Last modified April 24, 2015. unknown author.
- ↑ Acid Dissociation Constant Ka Chemistry Revision. Web. Published June 12, 2008. unknown author.
- ↑ Phenol Summary Pub Chem. Web. Last modified November 12, 2016. unknown author.
- ↑ Phenol Phenol. Web. Last accessed November 3, 2016. unknown author.
- ↑ Properties of Phenol UCC. Web. Last accessed November 13, 2016. unknown author.
- ↑ 6.0 6.1 6.2 6.3 Phenol Sussle Visual Encyclopedia. Web. Last accessed November 10, 2016. unknown author.
- ↑ 7.0 7.1 7.2 Phenol Chemical Compound Encyclopædia Britannica. Web. Last accessed November 13, 2016. unknown author.
- ↑ 8.0 8.1 Uses Of Phenol BYJU'S. Web. Published December 18, 2015. unknown author.
- ↑ Why Plastics Important ENVIS. Web. Last modified August 17, 2011. unknown author.
- ↑ AzoDye Encyclopædia Britannica. Web. Last accessed November 13, 2016. unknown author.
- ↑ The Nazi Doctor Medical Experiment. Web. Last accessed November 13, 2016. unknown author.
| ||||||||||||||




