Ununtrium
| Ununtrium | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

| |||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Uut | ||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::113 | ||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::286 g/mol | ||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Metal | ||||||||||||||||||||||||||||||||||||||||||
| Appearance | Solid 
| ||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 13,7,p | ||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 6d10 7s2 7p1 | ||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2,8,18,32,32,18,3 ? 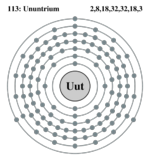
| ||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::54084-70-7 | ||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||
| Phase | Solid (predicted) | ||||||||||||||||||||||||||||||||||||||||||
| Density | Density::18 g·cm−3 (predicted) g/ml | ||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::700 K, 430 °C, 810°F (predicted) | ||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::1400 K, 1100 °C, 2000 °F (predicted) | ||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Ununtrium | |||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||
Ununtrium is a chemical element known by the chemical symbol Uut. It was discovered in 2003 using the cyclotron particle accelerator, resulting in only 4 atoms being successfully produced. Due to the minimal amount and failure of other labs to repeat the results using the same technique, the International Union of Pure and Applied Chemistry (IUPAC) did not confirm its discovery, leaving it with a temporary name holder.
Ununtrium is a super-heavy element which is highly radioactive. To date, there have six isotopes produced, which all decay by alpha decay. The most stable isotope has the mass number of 286.
Discovery
Ununtrium was first discovered in 2003, by Joint Institute for Nuclear Research in Dubna, Russia and the Lawrence Livermore National Laboratory (LLNL), California, U.S[1] . The collaboration of the two teams was led by the two scientists, Yuri Oganessian and Ken Moody. They believed that element Ununtrium did not exist in nature, but was produced through an experiment in their lab. The experiment took place within one month, starting from July 14, 2003 and ending on August 10, 2003. The scientists created Ununtrium by firing the element Calcium from a cyclotron, it would then hit element Americium in order to produce element Ununpentium, this then decay into element Ununtrium [1].
In 2004, a group from The Riken Nishina Center for Accelerator-based Science in Japan, led by Dr. Kosuke Morita, synthesized successfully and observed element Ununtrium [2]. This research was not confirmed to the public after nine years since its start. This is the first synthesized element produced in Japan [2]. However, they did not use the same concept as the LLNL. They did not synthesize it by bombarding the element Calcium (atomic number 20) and the element Americium (atomic number 243). Instead, they created a fusion of zinc ion (atomic number 30) with bismuth atoms (atomic number 83) in order to make the element Ununtrium [2]. Nuclear fusion is the process by which multiple atoms having the same charge join together in order to form a heavier nucleus [3] . Because this Japanese group did not gather enough information to prove it to the International Union of Pure and Applied Chemistry (IUPAC), Ununtrium was not approved as an official element.
However, in 2011, the International Union of Pure and Applied Chemistry (IUPAC) recreated the experiment; but they did not receive the same result. Because of the lacking information about its further properties, they required more evidences to accept the discovery of element Ununtrium. That's why the element Ununtrium is not appear on the periodic tables because they did not collect enough information. Some scientists have tried to recreate the experiment that was held by the LLNL to produce more atoms Ununtrium [1] . However, they did not receive the same record, such as the IUPAC. Because it was lacking some of the most important required sources, Ununtrium atoms, for the test, some of its physical and chemical properties were unknown.
Ununtrium is a temporary name until the IUPAC decides who will receive the credit for its discovery so they can name it after the true founder [4] . Its current name, Ununtrium, was a combination of words representing its meaning. “Un, un, tri” means “one, one, three” which is Ununtrium’s atomic number. Until now, element 113 has not receive any permanent name [4]. However, the Russian group who first discovered it, proposed to name it as “becquerelium” in honor of noted French physicist Henry Becquerel [4].Henry Becquerel was a French scientist who discovered radioactivity[5]. He placed a uranium-bearing crystal under the sunlight for a while [5], then, he placed the uranium-bearing crystal on a photographic plate [5], a flat sheet of metal or glass on which a photographic image can be recorded [6]. He observed that the crystal produced its image on the plate[5] . He concluded his experiment that the absorbed energy of the sun in the crystal was released in the form of x-rays [5]. He called this conclusion radioactivity. After the discovery of radioactivity, many other scientists including Marie Curie to describe the spontaneous emissions that they studied [5], and Ernest Rutherford used radioactivity in his experiment to figure out that atoms have a strong dense nucleus and electrons act as a cloud surrounding the nucleus [5]. Rutherford’s discovery objected previous observations of the atoms; it helpeed confirm the truth of the atom that can lead later scientists to walk on the right path.The Japanese group suggested the name “japonium”, which stand for Japan or “rikenium”, which stand for their institute name in honor of their own research [4]. There could be a chance that Ununtrium will not have a temporary name for a long time because same situations happened to other elements before [4].
Synthesis
Some heavy elements and super-heavy elements are produced artificially in cyclotron experiments [7]. Ununtrium is one of the elements that was produced by this method [7]. In July 14, 2003, the scientists from Joint Institute for Nuclear Research in Dubna, Russia and the Lawrence Livermore National Laboratory (LLNL), California, U.S cooperated to produce the element Ununtrium by using the cyclotron particle accelerator method [1]. In 1930, the scientist, Ernest Lawrence, successfully invented the cyclotron particle accelerator [8]. The first device that he made was only 4 inches in diameter [9]. His idea came up after he realized that the previous model of the particle accelerator machine, called the linear accelerator, required a vacuum tube many meters long [10]. He illustrated his idea that the new accelerator machine will have a bending circular path, using a magnetic field, in order to send the particles through the same electrode constantly [10]. The cyclotron has two separate “D” shapes magnetic field regions [11]. The cyclotron uses an electric field to accelerate charged particle across a gap between the two “D” shapes [11]. The electric field is extremely important because it help the charged particles to produce energy. An electric field with a frequency of about four million cycles per second lay in the region of the short waves[10]. When these waves were combined with the magnetic field of the “D” shapes, a potential on the conductor used to make electrical contact that provides a path for electrical current to flow could accelerate from ten thousands volts to one million electron volts [10]. The charged particle will be spinning in a semicircle around the magnetic field [11]. Whenever the charged particles enter the opposite “D” shapes, the electric magnetic field will be reversed so the charged particle will be rotated back to where it was [11]. This method helps the charged particle reach its highest energy. As the particles moved faster, the radius of the their circular path became bigger until they hit the target on the outermost circle[9]. Lawrence’s cyclotron could not produce high energy charged particles as modern day’s cyclotron [9], because of its lacking tools and techniques. However, he has put the foundation for the cyclotron for modern scientists to keep doing researching as the previous linear particle accelerator inspired Earnest Lawrence to come up with a more effective and useful cyclotron.
The scientists from the LLNL used cyclotron as equipment in their Ununtrium researching [12]. They formed Calcium ions inside a cyclotron in order for calcium to reach its most desirable energy. When Calcium gains enough energy, it will be automatically fired out of the cyclotron to shoot at a target layer Americium, deposited on titanium foil [12]. Four Dubnium atoms were produced which has the atomic number 115 [12]. Then, Ununtrium is made through alpha decay of Dubnium [12].
Properties
It is predicted that Ununtrium would have some of the same properties as element #81, Thallium or element #49, Indium because they are in the same family [13].According to Thallium’s character, “when it is heated strongly to red heat in air, poisonous thallium (I) oxide is formed” [14] . There is the chance that Ununtrium will produce a toxic element like Thallium; however, it is only a prediction. As similar to its reaction to air, Ununtrium’s reaction to water, halogens, acid and bases are also unknown.
Ununtrium is a radionuclide, which is an atom with an unstable or radioactive nucleus. [15]. A radionuclide will decay by emitting radiation, such as the alpha partice (a helium nuclei)[15] . When the element eventually decays to a stable element, the radiation will also stop emitting [15]. All the nucleus of all elements required the help of radioactive decay in order to reach the stable state. Ununtrium is an unstable element [4]. Usually, each element can be decayed by one of these four radioactive atoms decay ways, including alpha decay, negative beta emission, positron emission and electron capture [15]. Each type of decay will determine the ratio of neutrons to protons that whether it will be increase or decrease in its isotopes [15]. Since Ununtrium was produced in 2003, there are only fourteen atoms of Ununtrium that have been observed [16] which lead to the amount of limited information that can be used to study further of this element. However, the scientists got the chances to record its isotopes. Until now, there are six isotopes that have been recorded [17]. The most stable isotope is the nuclear has the mass number of 286, which has a half-life of 20 seconds. All of these six isotopes are decayed by alpha decay [17].
In alpha decay, the nucleus will exclude two protons and two neutrons out of the atom, also known as Helium element[15] ; this will reduce its atomic mass down by four and it will become a different element that has two less protons compared to the original [15]. The loss protons and neutrons will be converted into energy and escaped, according to the law of energy transformation [18]. Atoms that have a large chance of decaying won’t last long because they keep spontaneously produced its half-life atom [19]. In order for any radioactive atoms to decay, it will be involved with a half-life [15]. A half-life is the amount of time required for half of a radioactive atom to undergo a radioactive decay [15].; which it is also known as radioactive half-life or physical half-life [15].. For example, Ununtrium isotopes 278 take 0.24 milliseconds to decay half of the original atom [17]. It will take another 0.24 milliseconds to decay half of the remaining. It will continue decay half of the left-over like that until it reach its stable condition. A factor contributes to its unpredictable property is that half-life can vary from milliseconds to billions of years. Until now, six of the Ununtrium half-life atom that were observed range from the smallest is 0.24 half-life and the most stable is 20 seconds [17].
Heavy element is any element that has the atomic number larger than 92 [7] . Ununtrium is a heavy element because it has the atomic number 113. Some heavy elements are produced in nuclear reactors, and some are produced artificially in cyclotron experiments [7]. However, it would be more correct to call Ununtrium as a super-heavy element. A super heavy-element is any element that has the atomic number larger than 112 [7] . Ununtrium is the first element that belongs in this group because it has the atomic number of 113. Discovering new super-heavy element proves the long existence of nucleus due to the island of stability of super-heavy elements and the utmost boundary of the periodic table of elements [7]. The island of stability is a term that explains the possibility of elements with stable number of either protons or neutrons that arrange into complete shells within the atomic number [20]. It will help the isotopes atom reach its most stable state than other isotopes atom of the same element [20]. We could also understand it as the half-life of the isotopes atom will lasts longer. It will have the half-life vary from seconds or minutes while other atoms who did not reach the island of stability only lass from micro or nanoseconds [20].The discovery of a super-heavy element is really important because it help the scientists to study further how nuclear are held together and how they resist the fission process [7]. Fission is the state when nucleus split into two separate but smaller nuclear because of the collision of a particle or the operating at the same time of only heavy elements [21]. This study of super-heavy elements will help the scientists present and secure the safety for citizens due to the radioactive nuclear in nuclear stockpile [7].
Uses
Since the first time Ununtrium was discovered in 2003[1],there are only fourteen atoms of this element that have been observed [16]. Because of the absence of substantial Ununtrium atoms to do research, little is known about its properties. Another factor that contributes to its minimal circumscribed data is although many scientists from different labs have tried to recreate the experiment, they also received unexpected results. No scientists have successfully produced element 113 like the collaboration of the first two Russian and American teams, and the Japanese team who synthesized it[1]. Currently, Ununtrium had no uses in our livings, but the scientists still do many experiments to study further properties of Ununtrium.
Video
Video description here....
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Stewart, Doug. Ununtrium Element Facts Chemicool. Web. Accessed October 9 2013.
- ↑ 2.0 2.1 2.2 Dodson, Brian. Discovery of element 113 confirmed nine years after first detection Gizmag.Date-of-publication September 28 2012.
- ↑ Black, Ken. What is Fusion Wisegeek. Web. Date-of-publication Sep 12 2013.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 McMahon, Mary. Naming Ununtrium WiseGeek. Web. Date-of-publication September 14 2013.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 . The Discovery of Radioactivity Cruising Chemistry. Web. Accessed October 18 2013 Unknown.
- ↑ . photographic plate The free dictionary. Web. Accessed October 18 2013 Unknown.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 Shaughnessy, Dawn. Discoery of Element 113 and 115 Physical and Life Sciences Directorate. Accesed October 20 2013.
- ↑ Yarris, Lynn. Ernest Lawrence's Cyclotron: Invention for the Ages Berkeley. Web. Accessed October 21 2013.
- ↑ 9.0 9.1 9.2 Freudenrich, Craig. How Atom Smashers Work? How Stuff Works?. Web. Accessed October 21 2013.
- ↑ 10.0 10.1 10.2 10.3 . [http://www.aip.org/history/lawrence/epa.htm Early Particle Accelerator AIP. Web. Accessed October 21 2013 Unknown.
- ↑ 11.0 11.1 11.2 11.3 . Cyclotron Accelerators Hyperphysics. Accessed October 21 2013 Unknown.
- ↑ 12.0 12.1 12.2 12.3 Stewart, Doug Discovery of Ununtrium Chemicool. Web. Accessed October 22 1013.
- ↑ .Ununtrium properties webelements. Web. Accessed Octorber 9 2013 Unknown.
- ↑ . Thallium properties webelements. Web. Accessed Octorber 9 2013 Unknown.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 15.8 15.9 .Decay and Half-life IEM. Web. Accessed Octorber 16 2013 Unknown. Cite error: Invalid
<ref>tag; name "Decay" defined multiple times with different content Cite error: Invalid<ref>tag; name "Decay" defined multiple times with different content Cite error: Invalid<ref>tag; name "Decay" defined multiple times with different content Cite error: Invalid<ref>tag; name "Decay" defined multiple times with different content - ↑ 16.0 16.1 Ununtrium observed atom Princeton edu. Web. Acessed October 15th 2013 Unknown. Cite error: Invalid
<ref>tag; name "14atomsobserved" defined multiple times with different content - ↑ 17.0 17.1 17.2 17.3 Gagnon, Steve.[Isotopes of Elemants Ununtrium Jefferson Lab. Web. Accssed October 16 2013. Cite error: Invalid
<ref>tag; name "isotopes" defined multiple times with different content Cite error: Invalid<ref>tag; name "isotopes" defined multiple times with different content - ↑ . Energy TransformationsEnergy Home Accessed October 16 2013 Unknown.
- ↑ Koks, Don. What are half-lfe and mean life mean? Math Edu''Published May 2003.
- ↑ 20.0 20.1 20.2 . Island of Stability Princeton Edu. Accesed October 18 2013 Unknown.
- ↑ . Nuclear Fission European Nuclear Society. Web. Accessed October 20 2013 Unknown.
| ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||



