Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. Chemical bonds can be between atoms to form compounds (often referred to as atomic bonds), or between compounds (known as intermolecular forces. The formation of a chemical bond always releases energy. Once a bond is formed, that same amount of energy must be supplied to break the bond.[1]
The three types of atomic bonds that hold atoms together into compounds are covalent bonds, ionic bonds, and metallic bonds. Covalent bonds are the most common type of bond, usually between two nonmetals. Ionic bonds normally occur between a nonmetal and a metal. Lastly, metallic bonds happen between two metals. Metallic bonds are the most unique bonds and are quite different from the other bonds. Two molecules with different electronegativities (attractions for electrons) will usually take part in bonding because it could be easier for one to give up an electron and one to receive an electron.
Atomic Bonds
Covalent Bonds
- Main Article: Covalent bond
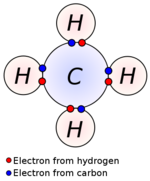
Covalent bonds are found both in molecules and in polyatomic ions. A covalent bond is produced by the joining of two atoms that essentially "share" electrons between them to complete their outer electron shell. A shared electron spends part of its time completing a stable electron configuration for each of the two atoms that share it. The bond may be single, double, or triple, which refers to the number of electrons that the two atoms share between them.
This type of intramolecular bonding mainly happens between two nonmetals or the same element, but can occur between a metal and a nonmetal. Covalent bonds take place between atoms that have similar electronegativities. Covalent bonds are hard to break because of their strong bonds.[2][3] The molecules that participate in covalent bonding are not very attracted to one another so they can move around each other freely. Most covalent bonds create gases or liquids.[4] The most basic covalent bond is between two hydrogens to create H2.
Another type of covalent bond is a polar covalent bond. A polar covalent bond is when two molecules share an electron pair, but the sharing is unequal. The electrons in the pair will orbit the more nonmetallic atom in a polar covalent bond. In this type of bond, one of the atoms will experience a positive charge while the other will experience a negative charge. This occurrence of one side being more positive and the other being more negative means a dipole moment will be produced.[5]
We come across many polar covalent bonds in our daily lives. One main way is H2O. In this case, the oxygen atom is the more negatively charged atom, and spends more time with the electrons, while the hydrogen atoms have a positive charge. HCl (hydrochloric acid) is another polar covalent bond in our lives. HCl is used in batteries and fireworks. In HCl, hydrogen once again is the more positive atom while chlorine has a negative charge.[3] Some compounds that have covalent bonds are: CH4, methane, CO, carbon monoxide, and IBr (iodine monobromide). When at room temperature, covalent bonds will exist as any of the three states of matter: solid, liquid, or gas. Covalent bonds do not conduct electricity when dissolved in water or in pure water. Covalent bonds have lower boiling points and melting points in comparison to ionic bonds.[6]
Covalent bonds are found both in molecules and in polyatomic ions. A covalent bond is produced by the joining of two atoms that essentially "share" electrons between them to complete their outer electron shell. A shared electron spends part of its time completing a stable electron configuration for each of the two atoms that share it. The bond may be single, double, or triple, which refers to the number of electrons that the two atoms share between them.
Ionic Bonds
- Main Article: Ionic bond
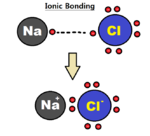
Ionic bonds or electrovalent bonds are also very common bonds. An ionic bond occurs when valence electrons are completely transferred between the two atoms. This type of bonding will result in two charged ions; one being positive and the other being negative. Ionic bonding usually takes place between a metal and a nonmetal.[2] The way these compounds are held together is through electrostatic attractions. The electrostatic attraction makes this bond very favorable.[7] These bonds are essentially a covalent bond but in an extreme case, rather than just sharing, there is a complete transfer of electrons.[8] Ionic bonds occur between two atoms with very different electronegativites.
Some different compounds that have ionic bonding are: NaCl, sodium chloride, KI, potassium iodide, and MgCl2, magnesium chloride. Unlike covalent compounds, ionic compounds will only exist as solids at room temperature. When dissolved in water, ionic compounds can conduct electricity, but are unable to as a solid. Because ionic compounds happen in stable crystalline structures they have high boiling points and melting points in comparison to covalent bonds.[6]
Metallic Bonds
- Main Article: Metallic bond
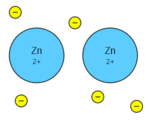
A metallic bond occurs between two metals. Metallic bonds are quite different from the covalent and ionic bonds. The main difference is that in ionic and covalent bonds are between two atoms, but metallic bonds form between many atoms. In metallic bonding, the atoms are positively charged with the valence electrons surrounding the atoms having a negative charge. Metallic bonds' properties are quite unique. Metals are ductile, which means the metal can be stretched. They are also malleable, meaning the metals can be made into different shapes, and they can conduct electricity and heat well.[9]
Electrons in these bonds move freely and nonlocalized. The way the electrons move is one main reason these bonds are capable of conducting electricity, and why they are malleable and ductile. The electrons act as buffers between the atoms in metallic bonds allowing the metals to be shaped differently.[9]
These bonds also tend to have lower ionization energies due to the free movement of the electrons. Metallic bonds are weaker bonds because of the attraction of electrons to many different atoms at once. This bond is non-directional meaning the charge of the bond is the same in every direction, much like the ionic bond. The strength of a metallic bond is in between the strength of a covalent and ionic bond which means its melting and boiling points are in between ionic and covalent bonds. Some examples of metallic bonds are Cu, copper, Al, aluminum, and Au, gold. [9]
Intermolecular Forces
Hydrogen bond
- Main Article: Hydrogen bond
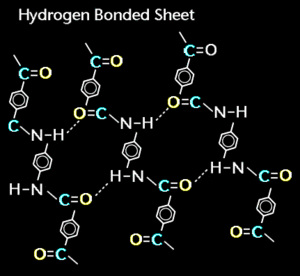
Hydrogen bonding is a weak bond that occurs when two electronegative atoms, such as oxygen and nitrogen, interact with the same hydrogen. This is also known as a strong form of intermolecular interaction. Hydrogen bonding is a strong form of intermolecular attraction. There are many elements that will combine with hydrogen to form compounds, in this case, also known as "hybrides". Hydrogen bonds, which are constantly being broken and reformed in water, have approximately 1/10 the strength of an average covalent bond. Every water molecule can potentially form four hydrogen bonds with the help of its surrounding water molecules. [1]
Dipole-Dipole Force
Dispersion Force
Video
Ionic, covalent and metallic bonds
References
- ↑ Brown, Lawrence S.; Holme, Thomas A (2011). Chemistry for Engineering Students (2nd ed.). Belmont, CA: Brooks/Cole. p. 208. ISBN 978-1-4390-4791-0.
- ↑ 2.0 2.1 Ionic and Covalent Bonds libretexts.org. Web. Accessed May 7, 2017. Unknown Author.
- ↑ 3.0 3.1 Polar and Nonpolar Covalent Bonds study.com. Web. Accessed May 7, 2017. Unknown Author.
- ↑ Covalent Bonding quatr.us. Web. Accessed May 7, 2017. Unknown Author.
- ↑ Chemical Bonds astr.gsu.edu. Web. Accessed May 7 2017. Unknown Author.
- ↑ 6.0 6.1 Comparison between Covalent and Ionic Compounds boudless.com. Web. Accessed May 7 2017. Unknown Author.
- ↑ Ionic Bonds chem.libretexts.org. Web. Accessed May 7 2017. Unknown Author.
- ↑ Ionic Bond britannica.com. Web. Accessed May 20 2017. Unknown Author
- ↑ 9.0 9.1 9.2 What is a Metallic Bond? study.com. Web. Accessed May 21, 2017. Unknown Author. Cite error: Invalid
<ref>tag; name "study-metal" defined multiple times with different content Cite error: Invalid<ref>tag; name "study-metal" defined multiple times with different content
| ||||||||||||||