Einsteinium
| Einsteinium | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Es | ||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::99 | ||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::252 g/mol | ||||||||||||||||||||||||||||||
| Chemical series | Actinide | ||||||||||||||||||||||||||||||
| Appearance | Metallic, silvery grey or white | ||||||||||||||||||||||||||||||
| Group, Period, Block | 3, 7, f | ||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f11, 7f2 | ||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 29, 8, 2 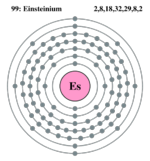
| ||||||||||||||||||||||||||||||
| CAS number | CAS number::7429-92-7 | ||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||
| Phase | Solid | ||||||||||||||||||||||||||||||
| Density | Density::8.84 g/ml | ||||||||||||||||||||||||||||||
| Melting point | [[Melting point::1133 K, 860 oC, 1580 oF]] | ||||||||||||||||||||||||||||||
| Boiling point | [[Boiling point::1130 K, 857 oC, 1574.6 oF]] | ||||||||||||||||||||||||||||||
| Isotopes of Einsteinium | |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||
Einsteinium is a chemical element named after the famous scientist, Albert Einstein. It is the seventh transuranium element, and one of the heaviest actinides.[1] It occurs nowhere in nature, and has no known uses outside of the research of the element itself.[2]Einsteinium was first identified in the fallout of the Ivy Mike nuclear test in 1952 by Albert Ghiroso and his colleagues.[3]
It has been subsequently synthesized, but only in small quantities as it decays rapidly. The half-lives of its isotopes vary anywhere from only thirteen seconds to about twenty days. The process of creating the element takes place inside nuclear reactors and requires several years, so the quantities attainable for research have been acquired at sparse intervals.[4]
Properties
Research of einsteinium is severely limited by its sparse availability, and the intense radiation of the decaying isotopes. Only several hundred milligrams of Es-253 can be produced, or made available, twice a year. The level of radiation emitted restricts the use of X-ray powder, which could otherwise be used for a bulk analysis of einsteinium compounds. Einsteinium is the seventh transuranium element discovered, and the heaviest actinide.[1]
The isotope Es-253 has a half life of roughly 20 days. Es-252 is the most stable isotope, with a half life of 471.7 days. Through alpha decay, Es-252 decays into berkelium-248. Though electron capture, it will decay into californium-252. Through beta decay, it will decay into fermium-252. Its ionization energy is 6.42 eV, with an oxidation state of +3. Isotopes of einsteinium vary greatly in their duration periods before undergoing alpha or beta decay. For example, einsteinium-241 has a half life of only eight seconds, undergoing alpha decay almost immediately. The half life of Es-242, Es-243 and Es-244 are 13.5, 19 and 37 seconds, respectively. Einsteinium-253 will occasionally undergo spontaneous fission. The half lives of Es-245, Es-246, Es-247, Es-248, Es-249, and Es-250 are 1.1 minutes, 7.7 minutes, 4.5 minutes, 27 minutes, 102.2 minutes, and 8.6 hours, respectively. Half lives of einsteinium's isotopes vary as their modes of decay, be they electron capture, alpha decay, or beta-minus decay.[5]
Discovery
Einsteinium was first discovered by Los Alamos Scientific Laboratory in the fallout from the Ivy Mike nuclear test in 1952 (the first thermonuclear device detonation). An isotope of Plutonium with a mass of 244 had been discovered in the fallout. The Pu-244 was found to have formed when six neutrons were instantaneously absorbed into uranium-238, which then underwent beta decay into the Pu-244 present after the reaction. The production of Pu-244 was originally planned to have taken place over the course of years, subjecting samples of plutonium to a high flux of neutrons. It was speculated that it would have taken many years before something like Pu-244 could be produced. Filter paper had been flown through the explosion cloud to be analyzed later. Albert Ghiorso, the man who first identified einsteinium, Stan Thompson, Ken Street, and Garry Higgins began to analyze filter paper they had received. At first, Ghiorso's supervisors were skeptical that U-238 could bind with as many as 16 neutrons. After analyzing the elution drops from a cation-exchange column, a high energy alpha particles at 6.6 MeV were detected in the results. At first, there was uncertainty whether this could be characteristic of the, then both theoretical, elements 99 or 100. To determine what the new element they were observing was, they added a Cf-246 tracer, a citric acid and ammonium buffer, to an acidic solution. Ghirorso and his colleagues were then able to determine the new element's atomic number, 99.[3]
Uses
There are so far no known applications of einsteinium outside of nuclear reactors and the research of einsteinium itself.[2]
Synthesis
Einsteinium does not occur naturally. Einsteinium has been synthesized in the past, and more recently by Oak Ridge National Laboratory. However, its production took over four years, starting with one kilogram of a plutonium isotope, and yielded only about 3mg of Einsteinium. In 1961, after the Ivy Mike tests, about .01mg of Es-253 was created. It was barely enough to separate out the einsteinium, and was measured using a magnetic balance.
Oak Ridge National Laboratory was able to synthesize 3mg of einsteinium by using samples of various plutonium isotopes, stored inside reactors. First, several kilograms of 239Pu were irradiated in a reactor for several years, until the Pu-239 had decayed into Pu-242. The Pu-242 was then formed into pellets, mixed with plutonium oxide and aluminum powder. The pellets were then loaded into target rods at the Savannah River Plant for a one-year irradiation. The rod samples then spent four more months in a High Flux Isotope Reactor. Once the samples are removed, they will contain californium and einsteinium. The einsteinium will exist in significant enough quantities, however, that it can be separated from the californium. [4]
References
- ↑ 1.0 1.1 Peterson, R.L., et al. (1979). Preparation, characterization, and decay of einsteinium(II) in the solid state Le Journal de Physique 40 (4):C4-111
- ↑ 2.0 2.1 [1] Chemistry Explained. Web. Accessed 16 November 2011. Author Unknown
- ↑ 3.0 3.1 Ghiorso, Albert. Einsteinium and Fermium Chemical and Engineering News. Web. 2003 .
- ↑ 4.0 4.1 [2] Radiochemistry.org. Web. Accessed 16 November 2011. Author Unknown.
- ↑ The Element Einsteinium Jefferson Lab. Web. Accessed 3 November 2011. Author Unknown.
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||



