Lead
| Lead | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Pb | ||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::82 | ||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::207.2 g/mol | ||||||||||||||||||||||||||||||
| Chemical series | Poor metals | ||||||||||||||||||||||||||||||
| Appearance | metallic 
| ||||||||||||||||||||||||||||||
| Group, Period, Block | 14, 6, p | ||||||||||||||||||||||||||||||
| Electron configuration | [Xe]4f14, 5d10,6s2,6p2 | ||||||||||||||||||||||||||||||
| Electrons per shell | 2,8,18,32,18,4 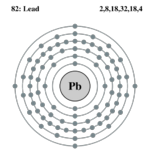
| ||||||||||||||||||||||||||||||
| CAS number | CAS number::7439-92-1 | ||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||
| Phase | Solid | ||||||||||||||||||||||||||||||
| Density | Density::11.34 g/ml | ||||||||||||||||||||||||||||||
| Melting point | Melting point::327.46°C | ||||||||||||||||||||||||||||||
| Boiling point | Boiling point::1749 °C | ||||||||||||||||||||||||||||||
| Isotopes of Lead | |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||
Lead is a chemical element that is classified as a poor metal and known by the chemical symbol Pb. It is a lustrous, bluish-gray or silvery-blue metal that is dense and somewhat soft. The color actually dulls when lead is exposed to the air. Lead is located in the fourteenth row, in the metallic section, of the periodic table; its atomic number is 82. Lead contains four natural stable isotopes, three that occur from the natural decay of thorium and uranium. It does not conduct heat or electricity, reacts slowly with hydrochloric acid, and readily with nitric acid. People used lead in ancient times, making it the oldest known element. Lead is very poisonous and if it is ingested it can cause serious health problems. The use of lead pipes to carry water causes lead to be absorbed into the blood stream. It can be found in its uncombined form in nature, and is distributed in the ores galena, cerussite, and anglesite, but its most commercial use is in lead-acid storage batteries. One interesting fact about lead is that the lead in lead pencils is not actually lead: it is graphite and clay. [1]
Properties
Lead, from the Latin word plumbum, has an atomic number of 82 and a density of 11.3437 g/cm3. The density of this element is higher than that of iron and copper, but less than mercury; it is the densest metal of all common and inexpensive metals. The isotopes of lead are Pb-204, Pb-206, Pb-207, and Pb-208. Pb-208 is the most abundant isotope at 52.3%. All the isotopes of lead are part of a natural radioactive series, except for the rare 1.48% abundance of isotope Pb-204. Lead melts at 327.35°C and boils 1515°C; only the elements tin and bismuth have lower melting points. [2]
Occurrences
Lead is found in various areas in nature and every modern day home. Lead enters the home through contaminated soil, lead pipes, and certain packaged food. Houses and buildings built before the 1950's may still be painted with paint with high lead content. Even the soil around these houses may be contaminated from years of lead paint, gasoline, or industrial usage. Some old houses, built around 1986, also have lead pipes that contaminate drinking water. This problem affects more modern homes as well. Lead enters into food and beverages during production, packaging and storage, and from certain natural calcium supplements, and imported items. Jewelry, computers, and some ceramic glasses that have been imported containing lead because none of the products are tested. [3]
Uses
Lead cab be found in pure metals, alloyed with other metals, and chemical compounds. Lead is used in car batteries, scuba weight belts, projectiles in fishing and firearms, paints, PVC plastic, sheathing material, organ pipes and so on. [4] Lead can also be used in storage batteries (the single most important commercial use), roofing and solder material. Lead oxide is commonly used in crystal glass manufacturing. Historically lead was quite useful in plumbing. Lead is no longer used because its environmentalists were concerned about the possibility of lead poisoning. [5] The main benefits of lead come from harnessing lead’s chemical properties. Lead’s density provides protection from radiation and lead stabilizers added to certain PVC products improve their durability, protecting underwater power and communication cables. Lead is also useful in hospitals, the dental industries, laboratories, and nuclear installations. [6]
Effects to Health
Lead poisoning is caused by tiny lead particles that contaminate homes and the environment. If a person swallows or inhales the lead particles the health risks can skyrocket, resulting in seizures or possibly even death. While lead poisoning is harmful to everyone, it is most harmful to children under the age of six because of the constant hand-to-hand contact. [7] In the environment the animals that are most affected by lead poisoning are ducks, geese, swans, and loons. The waterfowl ingest the lead from pellets, sinkers, or jig heads while they are feeding. The lead erodes while sitting in the gizzard or ventricles and slowly works its way into the circulatory system. From there the lead enters the animal's bones and exits through the feces. Just one pellet can cause anemia, and five doses result in heart attack or muscle paralysis. [8] There is no cure for lead poisoning, and the effects of it are irreversible. A blood test is the only way to identify if a child has lead poisoning. Low levels will not cause any known symptoms, but high levels of ingested lead particles cause death. Very high levels of lead poisoning have declined dramatically nationwide, and now lead poisoning rarely occurs in the United States. [9]
References
- ↑ Lead Author unknown. The Free Dictionary by Farlex. Accessed January 5, 2011.
- ↑ [1] Author unknown. Accessed January 5, 2011.
- ↑ [2] Author unknown. Accessed January 5, 2011.
- ↑ [3] Author unknown. Accessed January 5, 2011.
- ↑ [4] Author unknown. Accessed January 5, 2011.
- ↑ [5] Author unknown. Accessed January 5, 2011.
- ↑ [6] Author unknown. Accessed January 5, 2011.
- ↑ [7] Author unknown. Accessed January 5, 2011.
- ↑ [8] Author unknown. Accessed January 5, 2011.
| ||||||||||||||


