Lithium
| Lithium | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||
| General Info | |||||||||||||||||||
| Atomic Symbol | Atomic symbol::Li | ||||||||||||||||||
| Atomic Number | Atomic number::3 | ||||||||||||||||||
| Atomic Weight | Atomic weight::6.941 g/mol | ||||||||||||||||||
| Chemical series | Alkali metals | ||||||||||||||||||
| Appearance | silvery white/grey 
| ||||||||||||||||||
| Group, Period, Block | 1, 2, S | ||||||||||||||||||
| Electron configuration | 1s2 2s1 | ||||||||||||||||||
| Electrons per shell | 2, 1 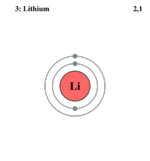
| ||||||||||||||||||
| CAS number | CAS number::7439-93-2 | ||||||||||||||||||
| Physical properties | |||||||||||||||||||
| Phase | solid | ||||||||||||||||||
| Density | Density::0.534 g/ml | ||||||||||||||||||
| Melting point | Melting point::180.54 °C | ||||||||||||||||||
| Boiling point | Boiling point::1347 °C | ||||||||||||||||||
| Isotopes of Lithium | |||||||||||||||||||
| |||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||
Lithium is a chemical elements known by the chemical symbol "Li". It is the lightest metal with an atomic number of 3, and the weight of 6.94, and generally has a soft and silvery-white color. Lithium has 3 electrons and 3 protons and is the least dense solid element. Lithium is in the group of alkali metals which are very reactive metals and that do not occur naturally. Lithium is malleable, ductile, and is a good conductor of heat and electricity.[1] Lithium receives its name from the Greek word lithos which means the stone. Due to the visual looks of lithium the name was given. The element is very useful in the medical fields. Doctors use to treat patients as drugs, vitamins, hormones, enzymes and also as a growth factors. Lithium was discovered as a treatment for mania and now is being used for many diseases and disorders. [2]
Properties
Lithium, the third element in the periodic table, is an active element but not as active as the other elements in the alkali metals. Lithium has one valence electron that gives up easily to make a cation. The reactions are very slow at a lower temperature and quicker at a higher temperature. Lithium, the lightest element from the metal are usually soft and silvery white colored. It has a very small amount of density about half as dense as water, but has the highest heat out of all the solid element.[3] Lithium reacts toward water like potassium and sodium but not as strong. Out of all the common metal lithium is the only element that reacts with nitrogen. It is usually placed in oil to not cause a reaction. Lithium is also very flammable and corrosive. It burns in crimson flame with the color of red but burns as a bright white color.It is a very good conductor of heat because it only has one valence electron.
Lithium can bond with hydrogen, nitrogen , sulfur, and the halogens to make different chemicals under certain circumstances. When they bond with oxygen they do not react at a room temperature but above a certain point they bond to make lithium oxide. [4] Lithium has a gravity of .534 and has a very low melting point of 356.97°F (180.54°C ) and a boiling point of 2,435°F(1,335°C).[3]
Occurrences
Lithium is very well distributed on earth but not found in native states or free in nature because of its high reactivity . It was first found in lithium-6 and lithium-7 which were the two forms of the isotopes and 65 parts per million lithium's are in the earth's crust. Now lithium is mainly found in igneous brine and rocks and even sometimes in compounds but there are very little that exists. It can also be founded from brine by evaporation at Searles Lake and Clayton Valley in United States.[4] This element a minor form of the rocks and mainly found in the natural brine's. It is more abundant than most of the elemnts in the periodic table.[5]
Lithium appears largely in the crust of the terrestrial. The main sources are from spudomene, lepidolite, petalite, amblyognite and eucrypitie. Spudomene can be found in North America, Spain and few other countries they are little ores that contain lithium. Lepiodolite and petalite can be found in Sweden and Africa but are not very common. Lithium has a low binding energies and atomic masses that causes to have less abundant amount of elements. [6] Lithium has many binary compounds that can also be formed, such as lithium hydride, lithium fluoride, lithium chloride, lithium chloride monohydrate, lithium iodide, dilithium oxide, dilithium peroxide, lithium superoxide, lithium sulphide, dilithium selenide, dilithium telluride, trilithium nitride, lithium iodide trihydrate and lithium hydroxide monohydrate. [7]
Uses
Lithium is very well known and can be use for multiple objects. Lithium can transfer heat due to its high specific heat. They are very light and durable due to the small amount of density. Lithium is mainly used to make special ceramics and glass. They can also be mixed with other elements to make a stronger foundation. Not only does lithium add strength to other elements, but it keeps the metals light weighted. [8] Lithium can be us to make lithium hydroxide which manufactures the greases, they can also make lithium-ion batteries and rechargeable batteries. Not only that but lithium niobate is essential in making phones they can easily combine with other metals to make the parts of an airplane. [9]
Lithium is used critically in medical fields. In the early 1800's lithium was used for people suffering from mania. Starting with that, lithium was a treatment for many other sufferings such as insomnia, cancer epilepsy and diabetes, although the uses of lithium decreased due to the lithium poisoning. After many trials, in the 1960s lithium was accepted to be a part of drug administrations. From that lithium is mainly used to help treat bipolar disorder. Lithium helps the patients to have a better control over their emotions and can help to cause less stress. Also lithium was very useful to treat patients with neutropenia and headaches.[10] Now lithium is still in use of many medical diseases such as cardiovascular, endocrine ,renal, dermatological, respiratory, pregnancy and lactation. Patients use lithium, should stop using lithium prior to their surgery so that they can restart at lower dose. Lithium also has many affects on glucose, patients who are using lithium treatment show the evidence of increased and decreased glucose tolerance. The studies have shown that lithium has effects on cell signals and reduces bronchial re-activity and is very useful for treatment of asthma. [10]
History
Lithium was first found as a chemical element by a Brazillian scientist José Bonifácio de Andrade e Silva in 1800, although it was not in use until Johan August Arfevdson and Jöns Jakob Berzelius worked together to discover the petallite ore in 1817, he gave the name lithos which meant stone in Greek. In 1818, Gmelin observe that with salt, lithium can create a bright red flame but he had failed to remove salt from lithium. In 1821, lithium was isolated by William Thomas Brande. [11] Then in mid 1800 lithium was recorded as a treatment of mania. The use of lithium in the medical fields was mainly from the Greek physician Galen. He treated his patients by making them drink water that contained lithium. Lithium became more popular in the medicinal purposes in 1843 when Alexander Ure put lithium to a modern medicine. Later Alfred Garrod discovered that other elements could be soluble in lithium and had made a bigger result in finding a cure for mania and depression. In 1840 lithium was dissolved with citrate and carbonate to treat diabetes, cancer, insomnia, and epilepsy. After many uses of lithium in the medicinal purposes, they had made tablets that helped tremor, diarrhea and vomiting. The drug disappeared later in 1949 to sever intoxication. [10]
References
- ↑ Lithium Element Facts. chemicool. Web. Accessed December 10, 2012 Author unknown.
- ↑ Lithium webmd. Web. Accessed December 10, 2012 Author unknown.
- ↑ 3.0 3.1 Helmenstine, Anne. Lithium Facts About. Web. Accessed November 25, 2012.
- ↑ 4.0 4.1 LITHIUM chemistry explained. Web. Accessed November 25, 2012 Author unknown.
- ↑ The economics of lithium Roskill. Web. Accessed November 26, 2012 Author unknown.
- ↑ Lithium - Discovery, Occurrence, Properties, Production and Applications of Lithium Azom. Web. July 19, 2011 Author unknown.
- ↑ Lithium Compounds webelements. Web. accessed December 9, 2012 Author unknown.
- ↑ The element Lithium education. Web. accessed November 26, 2012 Author unknown.
- ↑ The uses of lithium wanttoknowit. Web. accessed November 26, 2012 Author unknown.
- ↑ 10.0 10.1 10.2 Baker,Michael.Medical uses for lithium ehow. Web. accessed November 26, 2012. Cite error: Invalid
<ref>tag; name "medical" defined multiple times with different content - ↑ The History of Lithium mhi. Web. accessed December 9, 2012 Author unknown.
| ||||||||||||||
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||



