Xenon
| Xenon | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General Info | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Symbol | Atomic symbol::Xe | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Number | Atomic number::54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic Weight | Atomic weight::126.9 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical series | Noble gas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | 18, 5, p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 5s2, 4d10, 5p6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 8 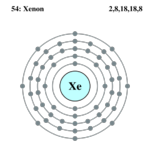
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | CAS number::7440-63-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | Gas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density | Density::5.894 g/ml | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Melting point::-111.7 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | Boiling point::-108.12 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of Xenon | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All properties are for STP unless otherwise stated. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Xenon is a chemical element with the symbol ("Xe"), and is member of the noble gases. In the early 1700s, people did not know the difference between the air and gases. In July 1892, Scottish chemist, Sir William Ramsay (1852-1916), and English chemist, Morris William Travers (1872-1961) discovered Xenon. Before the discovery of Xenon, Ramsay and Travers discovered krypton and neon which were the new technology of refrigeration. [1] The earth's atmosphere has about 0.0000087% of xenon. [2]
Properties
Xenon is a heavy gas which has no color, no taste, and no smell. This element is non-toxic. It is one of the inert and rare atmospheric gases. Xenon is a inert gas to most chemical reactions because the outer valence shell contains eight electrons.[3][4] The atomic radius of this gas is 1.24Å; atomic volume is 37.3cm3; covalent radius is 1.31Å. Xenon contains 54 electrons, 77 neutrons, and 54 protons. It has a boiling point of -108.12, melting point of -108.12, and density of 5.894 grams per liter. Xenon is a inert gas to most chemical reactions because the outer valence shell contains eight electrons.[5] Xenon has a much wider light spectrum than neon and Krypton. [6]
Occurrences
Xenon, a trace gas, is present in the Earth's atmosphere. This gas is rare in the Sun's atmosphere, on Earth, and in asteroids. Xenon occurs at 0.087±0.001 in one part in twenty million (μl/l). Xenon is found in gases produced from some some mineral springs. It has some radioactive species, 133Xe and 135Xe, and they are produced by neutron irradiation of fissionable material within nuclear reactors. [7]
Commercially, Xenon is obtained as a residual product of the separation of air into oxygen and nitrogen. After this separation, the liquid oxygen produced will contain small quantities of krypton and xenon. The liquid oxygen may be enriched to contain 0.1–0.2% of a krypton and xenon mixture by additional fractional distillation steps which is extracted either via adsorption onto silica gel. After all, this mixture may be separated into krypton and xenon via distillation. The extraction of xenon in the atmosphere needs 220 watt-hours of energy. Xenon is much more valuable than the lighter noble gases and, in 1999 in Europe, approximate prices for the purchase of small quantities were 10 €/L for xenon, 1 €/L for krypton, and 0.20 €/L for neon. [8]
Uses
Xenon is one of the noble gases. Most of them are used in lighting, for example, a neon sign. Xenon is mostly used in lamps such as Projection lamps, UV lamps, photographic flash lamps, stroboscopic lamps, and other powerful lamps. It also used in electron tubes, electronic flashes, bubble chambers and paint testers. [9][10]
Other noble gases
- Main Article: Noble gas
All the noble gases are colorless, odorless, and tasteless. They are present as monatomic gases, which means that their molecules are made up of a single atom. Noble gases make up about 1% of Earth's atmosphere collectively. Most of the noble gases have been discovered in small amounts in minerals and in meteorites.
The noble gases each have different uses and properties. Noble gases contain Argon(Ar), Helium(He), Neon(Ne), Radon(Rn), Krypton(Kr), and Xenon(Xe). Argon is used in light bulbs and it doesn't react with the metal filament. Helium is used to inflate the tires of large aircrafts and fill airships and weather balloons. This gas is non-flammable and has low density. Neon is used in advertising signs because when electricity passes, it glows red. Radon is known as the most dense gas. This gas is used in hydrologic research, geologic research, and to track air masses. Krypton and Xenon is used in many kinds of lamps and give out a lot of light when electricity passes through. [11]
References
- Element Xenon-Xe environmentalchemistry.com, 2/22/2007.
- Chemical Properties of Xenon www.lenntech.com
- Xenon Copyright © 2000. Corrosionsource.com, December 2005.
- Uses of Xenon www.c-f-c.com, 8/10/1998.
- Xenon, chemical element www.chemistryexplained.com, Copyright © 2009 Advameg, Inc.
- Properties, Applications and Uses of the "Rare Gases" Neon, Krypton and Xenon www.uigi.com, Universal Industrial Gases, Inc.
- The Element Xenon education.jlab.org
- Periodic Table: Xenon Yinon Bentor, www.chemicalelements.com, Copyright © 1996-2009.
- Xenon wikipedia.org, Wikimedia Foundation, Inc.
- Facts about Xenon www.facts-about.org.uk
- Xenon|Chemical elements summary www.bookrags.com
- Element facts:Xenon Alison Bowler, www.helium.com
- Characteristics of Xenon www.experiencefestival.com
- Noble gases www.scienceclarified.com, Copyright © 2009 Advameg, Inc.
| ||||||||||||||



