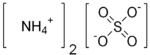Ammonium
| Ammonium | |
|---|---|
| |
| General | |
| Systematic name | Ammonium |
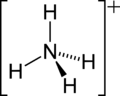
| Molecular formula | NH4+ |
| SMILES | [NH4+] |
| Molar mass | Molar mass::18.03851 g/mol |
| Appearance | Colorless gas |
| CAS number | CAS number::14798-03-9 |
| Properties | |
| Melting point | Melting point::−77.73 °C |
| Boiling point | Boiling point::−33.34 °C |
| Acidity (pKa) | 9.25 |
| Structure | |
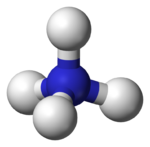
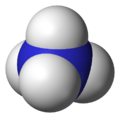
| Molecular shape | Tetrahedral |
| Dipole moment | 0 D |
| Related compounds | |
| Other cations |
Ammonium Sulfate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Ammonium is a polyatomic ion which can be created by mixing ammonia and Bronsted acid. Ammonium is a gas and it is harmless to human. Ammonium is a little acidic and a positively charged substituted amine. Ammonium's solubility, boiling point, or vapor pressures are predicted numbers but there isn't an exact number for solubility, boiling point and vapor pressures.
Properties
Ammonium is a polyatomic compound which can be created by mixing ammonia and bronsted acid. Ammonium is a gas and it is harmless to humans. most cations such as Ammonium sulfate or Ammonium phosphates are created by mixing Ammonia and the element that determines the name.[1] Ammonium's solubility, boiling point, or vapor pressures are predicted numbers aren't exact number for solubility, boiling point and vapor pressures. [2]
Synthesis
Ammonium is little acidic and positively charged substituted amines. A substituted amine is where one or more Hydrogen is replaced by organic groups. [3] When Ammonium ion is reacted with bronsted bases. it creates concentrated solution of Ammonium Nitrate and Ammonia. Also when Ammonia is mixed with the water, it creates little bit of Ammonium.[4]
Uses
It is impossible to use Ammonium alone, People usually mix it with other compound to create another, such as Ammonium Hydroxide, Ammonium Chloride, and Ammonium Nitrate. Ammonium Nitrate is used for High- Nitrogen fertilizer since it is very soluble in water and also because of Ammonium Nitrate's reaction it is used to make explosives, such as dynamite and most of the explosives in the United States. Ammonium Nitrate is not the compound you can achieve by mixing two different compounds because it's a natural mineral.[5] Another use of the Ammonium is Ammonium sulfate. People use Ammonium Sulfate as fertilizer which balances the acid level of the soil so soils become perfect condition for trees or any crop growth but People don't use them very often because of it's nature it is extremely hard to contain it and hard to transport it to buyers. [6] Ammonium Chloride is used for food ingredient. It is commonly called yeast and also used to make cookies crispy. Also squids oozy out Ammonium Chloride to float in the water.[7]
Different types of Ammonium
Ammonium Sulfate is mostly used for fertilize the soil. It contains about 21% of Nitrogen and 24% of Sulfur is added. It is used for fertilizing the soil because it balances the acid level in alkaline soil. Ammonium Sulfate is mostly used for Fertilization but not everyone uses it because it needs to be treated special way so it's way more expensive than the normal fertilizer.[8] Approximately, 90% of use of Ammonium Sulfate is fertilizer but other uses of Ammonium Sulfate is purifier. In laboratory, scientists use Ammonium Sulfate is used for purifying protein from the certain precipitation because Ammonium Sulfate is very soluble so it can make very concentrated solution, which action calls "salt-out" the protein. [9] Ammonium Sulfate is extremely dangerous to human and any creature with a respiratory system. If you inhale Ammonium Sulfate it will cause extreme irritation and inflammation in respiratory tract. Ammonium Sulfate won't kill you but will make human Vomit, nausea, and diarrhea, which may cause dehydration.[10]
Video
Jennifer Auh looks into ammonium nitrate, after the deadly explosion from a fertilizer plant in West, Texas.
References
- ↑ Unknown. Ammonium wikipedia. Web. Last modified 20 January 2015.
- ↑ Unknown. Ammonium ion Chemspider. Web. Accessed 27 January 2015.
- ↑ Unknown. Ammonium Wikipedia. Web. Last modified 20 January 2015.
- ↑ Unknown. Ammonium Wikipedia. Web. Last modified 20 January 2015.
- ↑ Unknown. Ammonium Wikipedia. Web. Last modified 1 December 2014.
- ↑ Unknown. Ammonium sulfate Wikipedia. Web. Last modified 8 January 2015.
- ↑ Unknown. Ammonium Chloride Wikipedia. Web. Last modified 9 December 2014.
- ↑ Unknown. Ammonium sulfate Wikipedia. Web. Last modified 8 January 2015.
- ↑ Unknown. ammonium sulfate Pubchem. Web. Accessed 13 January 2015 .
- ↑ Unknown. Ammonium Sulfate Aluminumsulfate.com. Web. Accessed 13 January 2015.
| ||||||||||||||