Citric acid
| Citric acid | |
|---|---|
| |
| General | |
| Systematic name | hydroxytricarboxylic acid |
| Other names | hydroxy propanetricarboxylic acid |
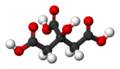
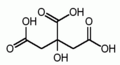
| Molecular formula | C6H8O7 |
| SMILES | C(C(=O)O)C(CC(=O)O)(C(=O)O)O |
| Molar mass | Molar mass::210.14 |
| Appearance | crystalline white solid |
| CAS number | CAS number::77-92-9 |
| Properties | |
| Density and phase | Density::166.5 g/ml |
| Solubility in water | 60g/100 ml (20°C) |
| Melting point | Melting point::153°C |
| Boiling point | Boiling point::175°C |
| Acidity (pKa) | 3.15 |
| Basicity (pKb) | 4.77 |
| Hazards | |
| Main hazards | alergic reation, erosion, painful, irritant |
| NFPA 704 | |
| Flash point | 100°C |
| R/S statement | R: 41 S: 26-39 |
| RTECS number | GE7350000 |
Citric acid is used in the process of metabolism in the humans, animals and plants. It is the product of a cyclic reaction in cellular respiration known as the citric acid cycle, and is an excellent source of energy because it can be readily converted into ATP. It is also used world wide as a artificial flavor for soda and other foods, for example citrus flavors.
History
Citric acid was first discovered in the 8th century by Arab-Yemeni alchemist, Jabir Ibn Hayyan. It was known to be in fruit by 13th century scholars and was recorded in the encyclopedia Speculum Majus or The Great Mirror, which was compiled by Vincent of Beauvias. Then it was rediscovered in 1784 by a Swedish chemist, Carl Wilhelm Scheele when he isolated it. In 1860 it was first used for industrial purposes and was based off the Italian citric fruit industry. C. Wehmer in 1893 discovered that penicillium mold from sugar can produce citric acid. Until World War 1 citric acid wasn't industrially important for microbial production. The American food chemist James Currie, in 1917, discovered that the mold, aspergillus niger, had certain strains that could be used for the production of citric acid. Two years later, Pfizer used the same technique in a industrial-level production.
Properties
Citric acid is a strong organic acid found in many fruits, vegetables, and is soluble in water. Citric acid's full name is hydroxytricarboxylic acid and it's chemical formula is six carbon, eight hydrogen, and seven oxygen. It is a colorless translucent crystalline acid that is a monohydrate and a anhydrous material. Citric acid is widely used since it is a nontoxic substance that is easily stored. In food it has a tart taste that is used in almost every commercial product, like soda for example.[1]Citric acid results from the three carboxide groups and it's ion is a citrate ion. Citrates can be buffers for metals because they can control the amount pH in acidic solutions. Citrates are used in the human body, in animals and plants.
Health Effects
Citric acid can cause many different allergic reactions in different people. If inhaled it may cause irritation in the respiratory tract and side effects include coughing and shortness in breath, go outside if symptoms continue to get fresh air or if not breathing call for medical attention. If ingested it might cause irritation to gastrointestinal tract and symptoms may include nausea, vomiting, and diarrhea. Extremely large doses may cause gastrointestinal disturbances and induce vomiting immediately as directed by a medical personnel in order to rid the person of the acid. Skin contact with citric acid may cause irritation of the skin including redness, itching, and pain. Immediately after contact on the skin, flush it under water for fifteen minutes and then remove all contaminated clothing, then get medical attention. If any citrus acid gets in the eyes it will cause irritation and might be abrasive. Immediately flush eyes for fifteen minutes with water and get medical attention. It also may cause tooth decay and erosion of enamel.[2]
Occurrences
Citric acid can be found in non-living and living things. Such as it is found in sweat, urine, animal tissue, and other body fluids. It is found in many fruits and vegetables, such as oranges, limes, lemons, grape fruit, tamarinds, goose berries, blue berries and cranberries or any other kind of berry. It is also found in the shell of eggs, fossils, proteins, milk, yeast, liquor, animals, cells, lichen, and any other plant matter.
Uses
Citric acid has many uses, for example it is used for flavoring of drinks and food in commercial items, used in dietary supplements, controls the pH in house hold cleaners and pharmaceuticals, soap and laundry detergents, regenerates ion exchange in water softeners, in the biotechnology and pharmaceutical industry to pacify the pipping in the lieu of nitric acid, a buffer of brown heroin, required for the synthesis of HMTD, added in ice cream for fat globules to not separate, recipes that include lemons or oranges or limes, blue prints, paper, drugs, cleans up sludge, extracting metals, before heart surgery, recycling waste, prevents the clotting in blood, narcotic, laxatives, algicides, animal feed, cigarettes, circuit boards, paint, concrete admixture, oil recovery, fertilizer, fossil fuel power plants, hair shampoos and cleansers, nuclear reactors, oil well acidizing, seafood, plating, ship bilge, reverse osmosis cleaning, canning industry, alcohol, skin toner, astringent, polishes metal, fermentation, cosmetic industry, and keeps oils from going rancid.
Citric Acid Cycle
The citric acid cycle has two other names that it is known by, the Krebs and the tricarboxylic cycle. The definition for the citric acid cycle generally is a series of chemicals reactions in a cell that breaks down food molecules into carbon dioxide, water, and energy. It is a central metabolic pathway that completes the oxidative degradation of metabolites in living things. The citric acid cycle has two main purposes it increases the cell's ATP producing potential and provides the cell with precursors that can be used to build a variety of molecules. Prokaryote cells and eurkaryotic cells both uses citric acid to function. Prokaryote cells use citric acid cycle for their cytosol and eurkaryotic cells use the citric acid cycle in the mitochondrial matrix.
There is eight different repeating steps in the cycle and it is one of the three stages of cellular respiration. The citric acid cycle begins with the glycolysis breaking down one glucose into two pyruvate, which produces six ATP's. The starting product, actetyl-CoA is made by the pyruvate. Every turn of the cycle oxidizes one pyruvate and every two turns it oxidizes one gluclose. Two turns will produce eight NADH's, two FADH2, and two ATP's. Then the NADH and FADH2 are oxidatively phosphorylated, which will result in twenty-eight ATP's. The three stages will in total have thirty to thirty-eight ATP's made in the citric acid cycle. [3] The eight steps are citrate, isocitrate, a-ketoglutarate, succinyl-CoA, succinate, fumarate, malate, and oxaloacetate. The more proper way to write it is Actetyl-CoA + 3 NAD+ + FAD + GDP + Pi + 2H2O <==> CoASH + 3 NADH + FADH2 + GTP + 2CO2 + 3H+.
References
- [4] Citric Acid (i.e. MSDS)
- [5] Uses (i.e. website)
- [6] Answers (i.e. website)
- [7] The Citric Acid Cycle (i.e. website)
- [8] Citric Acid (i.e. website)
| ||||||||||||||




