Glycine
| Glycine | |
|---|---|
| |
| General | |
| Systematic name | Aminoethanoic acid |
| Other names | Gly, Glycocoll, Sucre de Gelatine |
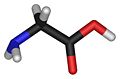
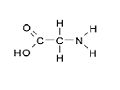
| Molecular formula | C2H5NO2 |
| SMILES | NCC(O)=O |
| Molar mass | Molar mass::75.07 g/mol |
| Appearance | White crystal powder |
| CAS number | CAS number::56-40-6 |
| Properties | |
| Solubility in water | 25 g/100 ml (25°C), 67.2 g/100 ml (100°C). |
| Melting Point | Melting point::248°C |
| Structure | |
| Crystal structure | NH2CH2COOH |
Glycine is the simplest of the 20 different amino acids used as building blocks to make proteins. Our body could produce glycine so we don't really need more from the food that we eat. And most of the people get 2 grams of glycine form the meals we have everyday. And there is no adverse effects form using glycine have been reported, even at doses as high as 60 g per day. One participant in the 22-person trial described above developed stomach upset and vomiting, but it ceased when the glycine was discontinued. [1]
Properties

Glycine is a sweet-tasting crystalline nonessential amino acid, that is the principal amino acid occurring in sugar cane. It is derived from the alkaline hydrolysis of gelatin and used in biochemical research and medicine.
Glycine is the smallest amino acids. Glycine could be appeared at both side of the molecule. The glycine will exist as the zwitterion when the pH value is at or near 7.[2]
The isoelectric point or isoelectric pH of glycine will be centered between the pKas of the two ionizable groups, the amino group and the carboxylic acid group.
Glycine could be thought as a derivative of the aminoethane. In estimating the pKa of a functional group, it is important to consider the molecule as a whole. Glycine is one kind of acetic acid, and the pKa of acetic acid is well known. Because of this, glycine could be considered as a derivative of aminoethane.
Function
Glycine could be used as a biosynthetic intermediate and a neurotransmitter.
As a biosynthetic glycine released into a synapse, and it binds to a receptor. And this binding makes the post-synaptic membrane more permeable to Cl- ion.[3]
In vertebrate animals glycine are being a neurotransmitter, it nullificated the synapse by a simple process of re-absorption by active transport back into the pre-synaptic membrane.[4]
Uses
The medicines that contain a high amount of glycine could augment the effectiveness of its uses in mental diseases like schizophrenia.
Some researchers are doing research on stroke. They gave the participants 1 to 2 g of glycine sublingually (dissolved under the tongue) or placebo treatment for a period of 5 days. The results of the research suggest that glycine can prevent neural damage. This proofed a guess about that glycine could protect against the spreading damage to the brain which usually follows a stroke. Although we already get a good answer for the research, but we still need a further research on the uses of glycine. [5]
Because of the study of the stroke, people thought that the glycine maybe increased the chance that the brain get injured. So the drugs that could prevent glycine bring damage to the brain have been developed. And this made an argument between whether or not the effect of glycine is positive.
Glycine has a sweet taste, so it was also used as sugar by the people has diabetes.
Other

There are a lot of natural foods that contain a high amount of glycine which is need by our body to biosynthesis the acids. Like nucleic acids and bile acids: Vegetable and Vegetable Products, Nut and Seed Products, Legumes and Legume Products, Meat Products, Finfish and Shellfish Products, Dairy and Egg Products, Sausages and Luncheon Meats, etc. [6]
 Browse |
References
- Glycine by Wikipedia
- Glycine G by The University of Arizona
- Glycine by Drugs & Supplements
- Glycine by Aurora Health Care
- Picture This by Pacific Northwest National Laboratory
- Brain Neurotraansmitters by Ben Best
| ||||||||||||||

