Sulfuric acid
| Sulfuric acid | |
|---|---|
| |
| General | |
| Systematic name | Sulfuric acid |
| Other names | Oil of vitriol Battery acid Dihydrogen sulfate Electrolyte acid Hydrogen sulfate Mattling acid Spirit of sulfur Sulphuric acid Acide sulfurique |
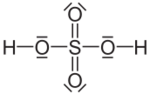
| Molecular formula | H2SO4 |
| Molar mass | Molar mass::98.086 g/mol |
| Appearance | Colorless, odorless, clear |
| CAS number | CAS number::7664-93-9 |
| Properties | |
| Density and phase | Density::1.84 g/ml, solid, liquid, gas |
| Solubility in water | Miscible |
| Melting point | Melting point::10°C |
| Boiling point | Boiling point::337°C |
| Acidity (pKa) | -3 |
| Viscosity | 26.7 cP (20°C) |
| Structure | |
| Molecular shape | Tetrahedral |
| Hazards | |
| MSDS | External MSDS Data Sheet |
| Main hazards | Toxic (T) Corrosive (C) Dangerous for the environment (N) |
| NFPA 704 | |
| Flash point | Non-flammable |
| R/S statement | R: R35 S: (S1/2), S26, S30, S45 |
| RTECS number | WS5600000 |
| Related compounds | |
| Related strong acids | Selenic acid Hydrochloric acid Nitric acid |
| Related compounds | Sulfurous acid Peroxymonosulfuric acid Sulfur trioxide Oleum |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Sulfuric acid, H2SO4, is one of the most (or perhaps the most) significant compound or acid on the earth. It is colorles, odorless, and oily liquid. It composes of two hydrogen molecules, one sulfur molecule, and four oxygen molecules. Sixty-five percent of all fertilizer products in markets come from a mixture of sulfuric acid and other chemical substances. In fact, it is the most manufactured compound in the world; in the United States, more than forty million tons of the acid are produced annually.[1] Although sulfuric acid is one of the most significant substances in the world today, it was only a little known in the sixteenth century. It was first discovered in the sixteenth century by a early Dutch-Flemist chemist, physiologist, and physician Johann Van Helmont. Van Helmont first named this substance the gas.[2] Sulfuric acid is also renowned as one of the most dangerous and powerful substances. The acid reacts the most to water. Due to its extremely corrosive characteristic, this acid cannot be found alone in the nature. Sulfuric acid can be produced either through lead chamber process or contact process.[3] One of sulfuric acid's most important uses is the dehydration process during which the acid's characteristic is utilized to remove water from a substance.[4] One must follow a MSDS instruction (or some kind of instructions) in a sulfuric acid experiment. Acid rain is the most renowned occurrence that contains sulfuric acid.[5]
Creation and the beginning of chemistry - water
- Genesis 1:1-8. "In the beginning God created the heavens and the earth. Now the earth was formless and empty, darkness was over the surface of the deep, and the Spirit of God was hovering over the waters. And God said, 'Let there be light,' and there was light. God saw that the light was good, and he separated the light from the darkness. God called the light “day,” and the darkness he called 'night.' And there was evening, and there was morning—the first day. And God said, 'Let there be an expanse between the waters to separate water from water.' So God made the expanse and separated the water under the expanse from the water above it. And it was so. God called the expanse 'sky.' And there was evening, and there was morning—the second day.[6]
Properties
Physical Properties
Physical properties of sulfuric acid result from the position and characteristics of its particles and that can be measured without causing a change in the identity of the material.[7]
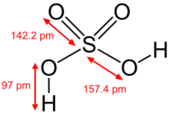
Sulfuric acid exists in liquid phase. The acid, in liquid form, is clear, colorless, odorless, viscous, yet extremely corrosive. The molar mass, which is the combined masses of its atoms, is 98.086 g/mol. The boiling point, which describes the temperature at which the vapor pressure of a liquid equals the applied atmospheric pressure [8], can measure up to 337 °C (or 610.15 K, 638.6 °F, or 1,098.27 °R). The melting point expounds a physical change from the liquid state to the solid state of sulfuric acid, and it is 10 °C, which can be converted as 283.15 K, 50 °F, or 509.67 °R [9]. Sulfuric acid has a density of 1.84 g/ml. Density can be explained as a measure of the concentration of matter; it is expressed as a ratio of the object’s mass to its volume [10]. The molecular shape of this compound is tetrahedral, a structural arrangement in which four particles surrounding a central particle are oriented toward the corners of a four-sided pyramid. Pure sulfuric acid is known to have approximately twice as density as water. Sulfuric acid has vapor pressure of 145.8 °C (295 F). Vapor pressure shows the acid’s pressure exerted by a vapor in equilibrium with its solid or liquid state at a specified temperature. Viscosity refers to the ability of sulfuric acid to resists flowing (amount of internal resistance; sulfuric acid has 26.7 cP (or 20°C) of viscosity. [11] [12] [13]
Chemical Properties
The chemical properties of sulfuric acid are those that describe how one substance reacts in the presence of other substances [14].
Sulfuric acid consists of hydrogen and oxygen molecules. It is also known as oil of vitriol, battery acid, dihydrogen sulfate, electrolyte acid, hydrogen sulfate, mattling acid, spirit of sulfur, sulphuric acid, and acide sulfurique, etc. This compound has a charge of zero, meaning it is electrically balanced. It is miscible with water, and releases much heat. The acid is one of the most renowned compounds in the world and it is often used to remove water due to its great reaction to water. Sulfuric acid is extremely hazardous, toxic, and harmful, and the most corrosive in its liquid form. [15] [16]
Sulfuric acid chemical reactions:
- Production of sulfur dioxide:
S8 + 8O2 → 8SO2
4FeS2 + 11O2 → 2FeO3 + 8SO2 [16]
- Sulfuric acid reaction with metals:
Cu + 2H2SO4 → CuSO4 + 2H2O + SO2
Zn + 2H2SO4 → ZnSO4 + 2H2O + SO2 [15]
- Reaction with metals:
C + 2H2SO4 → CO2 + 2H2O + SO2 [15] S + 2H2SO4 → 3 SO2 → 2H2O 2P + 5H2SO4 → 2H3PO4 + 2H2O + SO2 [15]
- Reaction with Alkalies
NaOH + H2SO4 → NaHSO4 + H2O
2NaOH + H2SO4 → Na2SO4 + 2H2O [15]
- Dehydrating agent
C11H22O11 → 12C + 11H2O [17]
History
Sulfuric acid has a formula H2SO4 (hydrogen sulfate), a compound of two hydrogen molecules and four molecules of oxygen. Despite the fact that this compound is significantly one of the most manufactured and widely used chemicals, it is assumed to be little known before the sixteenth century. [2]
Johann Van Helmont, a early Dutch-Flemist chemist, physiologist, and physician from a noble family, is considered first to have found this acid during the sixteenth century. Van Helmont, the one who also originally recognized and characterized a substance, naming it the “gas”, first prepared sulfuric acid by destructive decontamination of green vitriol (also known as ferrous sulfate) by burning sulfur. The Leblanc process, the industrial process for the making of sodium carbonate, became the first major industrial demand for sulfuric acid in the early eighteenth century. However, sulfuric acid produced in Nordhausen, Germany, from green vitriol was very expensive. [2]
Van Helmont is best known for his tree experiment in which he planted a tree in a pot of the soil, weighing both the tree and the tree after about five years. Fascinatingly, the soil weighed about few ounces lighter while the tree increased by over hundred pounds. The conclusion of this result, Van Helmont stated, was that the tree obtained water even though the anything else was not prepared. [18]
Johann Glauber, German-Dutch chemist and alchemist, first prepared an operation during which he burnt sulfur with saltpeter, or potassium nitrate in the early seventeenth century. Eventually, the process was examined and developed commercially by Joshua Ward from England during the late seventeenth century. John Roebuck, from England, furthermore improved this operation, superseding it by the lead chamber process. Since scientific development of the process by Roebuck, many others have ameliorated this experiment. [2]
Peregrine Phillips, an English vinegar merchant, first invented the process in 1830s later known to be called the contact process, wherein the pure sulfur dioxide and air are mixed. This process was not carried out widely until the demand for concentrated acid increased, mainly for the manufacture of synthetic organic dyes. In fact, the contact process, newer than the lead chamber, produces more concentrated and purer acid. [19]
Manufacturing
Sulfuric acid, with a formula H2SO4, is one of the most dangerous and powerful substances. Because of its great reaction to water, pure sulfuric acid cannot be found by itself on the planet Earth. This substance is extremely hazardous, toxic, and harmful, yet colorless, transparent, and odorless. The acid is viscous and very corrosive in its liquid form. In addition to its powerful acidic characteristic, it is the most manufactured acid among all the industrial chemicals. [3]
Sulfuric acid can be produced through either two processes: the lead chamber process, and the contact process. Concentrations and qualities, grades, etc, of the acid is adjustable, depending on the demands of the buyers. In both processes, sulfur dioxide is oxidized and dissolved in water. There are several approaches to obtain sulfur dioxide: burning sulfur, burning pyrites (also known as iron sulfides), roasting nonferrous sulfide ores preparatory to smelting, and by burning hydrogen sulfide gas. [3]
Lead chamber process
The lead chamber process, invented before the contact process, is mainly performed to make sulfuric acid for the production of fertilizers. First, it is required to wash a reactor called Glover tower with nitrous vitriol (containing sulfuric acid with nitric oxide, and nitrogen dioxide) and mixed with nitric oxide and nitrogen dioxide gases. Next, hot sulfur dioxide gas goes into the bottom of the reactor. A chemical reaction then occurs that oxidizes some of the sulfur dioxide to sulfur trioxide, and the fusion of the trioxide and the acid on the bottom of the tower forms an acid called tower acid or Glover acid (approximately seventy-eight percent of H2SO4). Then the mixture of gases (including sulfur dioxide and trioxide, nitrogen oxides, nitrogen, oxygen, and steam) moves to a lead-lined chamber to react the mixture with more water. The chamber can resemble a large, boxlike room, and can possess anywhere from three to twelve chambers in a series (gases passing through each of them successfully). After a series of complex chemical reactions, sulfuric acid is finally formed. The signal for the formation of the sulfuric acid occurs when the acid condenses on the walls of the Glover tower and aggregates on the bottom of the chamber. The resultant acid, known as chamber acid or fertilizer acid, contains sixty-two percent to sixty-eight percent H2SO4. The final chamber, called the Gay-Lussac tower, is a reactor that is washed with cooled concentrated acid, from the Glover tower in the beginning. As the gases pass through the chambers successfully and enter the final chamber, the nitrogen oxides along with unreacted sulfur dioxide dissolve in the acid to develop into the nitrous vitriol used in the Glover tower. The scientists generally dispatch the remaining waste gases into the atmosphere.
[20]
Contact process
Contact process is newer and much simpler of the two processes. Despite of the fact that it produces more concentrated acid, it requires purer raw materials and the use of expensive catalysts. In the contact process, the pure sulfur dioxide and air (H2) are mixed. It is then heated to about 450°C, and passes over a catalyst (usually platinum on a silica or asbestos, etc). The sulfur dioxide is oxidized to sulfur trioxide, which is cooled and passed through two towers (the first tower is washed with oleum, fuming 100 percent sulfuric acid, and the second tower is washed with ninety-seven percent sulfuric acid). Ninety-eight percent of the resultant sulfuric acid is manufactured in this second tower. Remaining waste gases are commonly released into the atmosphere.
Additionally, sulfuric acid can also be obtained from ferrous sulfate waste solutions from cleaning iron and steel, and also from waste acid mud from oil refineries. [20]
Uses
Sulfuric acid is considered as one of the most essential industrial chemicals, or compounds, and on top of that, it is one of the least expensive acids. In fact, it is the most manufactured chemical per year. In 1990, the scientists recorded that more than 100 billion pounds (that is, 45 billion kilograms or more) of sulfuric acid were produced only in the United States because of the compound’s significant uses. [21]
The acid has a variety of uses, extremely integral that it is practically utilized in all manufactured products. The compound also behaves as a catalyst, meaning it speeds up chemical reactions. One of the most significant uses of the acid includes the production of fertilizers, such as superphosphate of lime and ammonium sulfate. Remarkably, this acid can be fused with phosphate rock to create water soluble phosphates, which are vital for plant growth and survival. Sulfuric acid, not only used in fertilizers, but is also extensively used in the production of chemicals, etc; it is used in hydrochloric acid, sulfate salts, synthetic detergents, nitric acid, explosives, phosphoric acid, ether, plastics, metal sulfates, copper sulfate, chromium chemicals, rubber, film, paints, food containers, wood preservatives, soaps, pharmaceutical products, pulp, paper, cellophane, perfumes, disinfectants, dyes and pigments, even glue, and drugs. The explosives include nitroglycerin acid, and even tri-nitro-toluene (TNT), etc. [21]
- Manufacture of ammonium sulfate fertilizer
2NH3 + H2SO4 → (NH4)2SO4 [17]
- Manufacture of superphosphate fertilizer:
Ca3P(O4)2 + 2H2SO4 + 4H2 → Ca(H2PO4)2 + 2CaSO4 · 2H2O [17]
Moreover, the scientists frequently put this compound in petroleum refinery (also known as oil refinery) in order to wash contaminates out of gasoline and other industrial products. Petroleum refinery is a machine or installation that makes finished petroleum products from chemicals and mixtures, such as unfinished oils, natural gas liquids, crude oil, other hydrocarbons, and alcohol. [22]
Sulfuric acid uses also include removal of oxides from iron and steel before plating or galvanizing them. Rayon, an artificial silk-like fiber, contains this acid. Sulfuric acid serves as the electrolyte in lead acid batteries (the lead-acid storage battery commonly used in motor vehicles). The acid batteries normally have 33% H2SO4 (sulfuric acid), and estimated gravity of 1.25. Since sulfuric acid is extremely dehydrating, it is often used to remove water from other substances. [21]
Acid Rain
- Main Article: Acid rain
Acid rain (or officially termed as acid decomposition) describes several ways that acids precipitate from the atmosphere. The acid decomposition consists of wet and dry components. It is harmful and affects many creatures, including plants and animals. Acid rain is a direct method that the atmosphere uses to clean itself. Any form of precipitation with high concentration of nitric and sulfuric acids can be called acid rain – falling down from the atmosphere in the form of snow, fog, and dry materials, etc. According to scientific experiments, the depositions have higher amounts of nitric and sulfuric acid than those of normal substances. [5]
Acid rain formation is, in fact, induced by natural sources. Rotting vegetation and erupting volcanoes discharge some chemical substances that can engender acid rain; however, humans are mostly responsible for the cause. Human activities, especially the burning of fossil fuels by coal-burning power plants, factories, and automobiles, are the main source of acid rain. [5]
When these released gaseous substances undergo a series of chemical reactions in the atmosphere with water, oxygen, and other chemicals, the clouds capture suspended acidic particles and gases in the atmosphere and eventually form a legion of acidic compounds with water. The product of this chemical reaction is the conversion of sulfur oxides and nitrogen oxides into solution of sulfuric acid and nitric acid: [23]
SO2 + HOH → H2SO3
2 NO2 + HOH → HNO2 + HNO3
Plants become the sources to release sulfur dioxide and nitrogen oxides. After the releasing of the acid, the wind blows these compounds across the state and borders, possibly over hundreds of miles away. [5]
Wet Deposition
The term wet deposition refers to acidic rain, fog, and snow. The prevailing winds carry sulfur acid and nitrogen acid to a variety of areas. If these acids reach to areas where the weather is wet, the acids can precipitate to the earth as rain, snow, fog, or mist. These acidic substances are harmful and affect many plants and animals. Factors such as buffering capacity of the soils, various types of sea creatures, arboreal plants, and anything that influence or rely on the water largely influence the strength of the acid rain. [5]
Dry Deposition
On the other hand, the winds blow the acid to the dry areas. When the acid precipitates in the dry areas, it falls in the form of dust or smoke. This process is called the dry deposition. These precipitated substances stick to the ground, homes, automobiles, plants, animals, statues, and buildings, etc. In order for this dry deposited gases and particles to be eradicated, rainstorms are required, which frequently lead to increased runoff. However, the further problem lies; this runoff water renders the acid more acidic and harmful. [5]
Natural Rain
Even normal rainfall has scientifically been substantiated to be acidic to some extent due to the presence of dissolved carbonic acid, which can be obtained in soda pop. Scientists have recorded the pH level of the normal rain to be 5.6 to a low of 4.5 with the average of 5.0. [23]
Facts:
- Water droplets collected from the base of clouds in the Eastern U.S.during the summer have an average pH of 3.6, with some values as low as pH 2.6.
- The pH in the upper portion of a cloud is much higher. The final rain droplet has an average pH of 4.2 in the Northeast U.S.
- In Los Angeles, the pH of fog has been measured at 2.0 - about the acidity of lemon juice. [23]
Effects on Sea
All rainwater contains acidity. Potential of hydrogen, abbreviated as pH, measures the level of acidity in a substance from 0-14 with 7 being neutral. The lower number on the scale indicates more acidic. Acid rain has pH of below 5.6. [24]
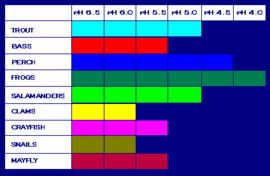
Acid deposition is extremely harmful to the environment and also to the living creatures. Although it affects both the life on the land and in the sea, it damages more in the ocean because the acid greatly contaminates the water and the fish need the water to survive. Thus, the oceanic creatures as well as marine frogs and insects perish as the acidity becomes pH of below 4.5. In fact, all the bacterial decomposers, animals, etc begin to die at a pH of 5.5. When these die, it eventually contaminates the water, which the fish need to live. Aquatic shrimp begin to wither as it hits 6.0. Oceanic plants grow the best when the acidity is between 7.0 and 9.2, but inaugurate to atrophy and die when the acidity becomes worse. Some of the lakes on the planet Earth have been acidified, but only few are recovering. The scientists have stated that only 33 % of the 202 lakes (that is about 66 lakes) have tuned out to be less acidic. [24]
Effects on the forests

Acid rain affects plants, especially trees. In the places where acid rain is common, such as Germany, the number of forests has decreased. The rain harms the arboreal waxy substances that surround and protect the leaves. As the substances are destroyed, the acid starts entering the tree. Plants undergo photosynthesis process to survive; however, the acid rain hampers them from consuming carbon dioxide to perform the process, and the plants eventually perish. The acid rain contains toxic metal substances that can destroy the green. The metals can be lead, zinc, copper, chromium, and aluminum. [24]
Effects on human lives
Acid rain can mainly harm us through our health. It has been recorded that some children and adults who have had some kind of breathing or lung problems (such as asthma) have been linked to acid air pollution. Everything in our lives: food, drinks, and air, etc, have once been in contact with acid deposits. However, if the product contains abnormal concentration of acid, it can affect us. Each year in the United States and Canada, acid rain causes heath problems: [24]
- 550 premature deaths
- 1520 emergency room visits
- 210,070 asthma symptom days.
Effects on objects
When acid rain affects non-living objects, such as statues, it decays its materials and paints. The rain can ruin important statues, buildings, or sculptures as well, things that commemorate certain events or people, etc. [24]
Pollution sources
| Air Pollutant | Mobile Resources | Other Resources |
|---|---|---|
| Volatile organic compound | 37% | 63% |
| Nitrogen oxide | 49% | 51% |
| Carbon monoxide | 81% | 19% |
| Particulate matter | 27% | 73% |
As the table shows, carbon monoxide is the biggest air contaminant that contributes to acid rain with 81 percent of the mobile sources. Particulate matter (little particles of pollution that automobiles discharge), is recorded to be the biggest other source. The particulate matter, with 73 percent contribution, can be burning diesel fuel, fertilizers, pesticides, road construction, steel making, and mining. If the world becomes economical, the occurrence of acid rain will not be as common. However, it is not an easy task to perform; we need cars and airplanes to get to places, and construction processors to develop the cities. [24]
In order to help reduce the pollution, we can:
- Only run the dishwater and the washing machine with a full load
- Turn off the lights when not needed
- Turn off the hot water tank when not needed
- Turn down or turn off the heat when not needed
- Use fluorescent light bulbs instead of incandescent light bulbs
- Recycle
- Stop burning things with fire
- Cover the pool with the pool cover when not used
- Walk instead of driving a car, or take a bus or train
- Avoid long trip in a car
- Make sure there is not a leak in your vehicle’s air conditioning system
- Remember not to overflow the gas tank
- Drive fast only when needed
Basic Management
- Humans intake sulfuric acid mostly via their respiratory system - by inhaling. This severely damages their lung.
- Sulfuric acid causes skin problems, such as chemical burn, severe pain, redness of skin, blisters, and nectrosis. The severeness depends on the concentration of the substance.
- Sulfuric acid can damage the human's eyes. When a person contacts with his eyes, sulfuric acid can irritate the eyes. This can cause burning, swelling, and tearing of the eys. In addition, it can diminish eyesight, and possibily cause blindness.
- Humans can consume sulfuric acid by ingesting. When that takes place, the mouth and throat start to burn. Also, it can cause severe pain in the chest and abdomen, followed by nausea and vomiting. It is possible for the esophagus to be severely damaged as well.[25]
Proper Handling
One must be well-acquainted with handling of sulfuric acid, etc. Sulfuric acid must be kept in a cool, dry, well-ventilated container. One must make sure the acid does not make any contact with the weather, extreme temperature changes, and physical damage. Due to its destructive characteristic, the acid, when making a contact with organic materials and metals, can cause fire as well as explosions, etc. If any undesired reaction occurs (fire, for an example), one must remove them if it can be done easily and safely. If it seems impossible, the use of dry chemical or carbon dioxide can extinguish the fire. This, however, only applies for small flames. Large fires must be extinguished with water. One must be careful when splashing the water because the reaction between these two substances (water and the acid) can cause some harmful reactions. It is important to prepare positive-pressure breathing appartatus in case of emit of toxic fumes. [25]
For more information, visit http://www.epi.state.nc.us/epi/oii/sulfuricacid/
Concentrated Sulfuric Acid
Sulfuric acid is one of the most significant compounds in the world for industrial purposes. It is extremely corrosive and thus classified as hazardous chemical. The hazard further intensifies as it increases its concentration. The more concentrated the sulfuric acid is, the more hazardous the substance it is. Accordingly, it is required to ascertain the factors of sulfuric acid and concentrated sulfuric acid. The information can be obtained from MSDS. [4]
The pure 100 percent acid, when heated to a certain point, loses sulfur trioxide gas (SO3). The substance, as it is heated to 337 C, reacts to be azeotrope that contains estimated of 98.5 percent sulfuric acid. Sulfuric acid, in fact, is one of the weak acids that have poor electrolyte. Because cold temperature reduces the movement of the kinetic energy contained within sulfuric acid molecules, it does not easily even react with iron or copper, the two common metals. However, on the other hand, when it is hot, sulfuric acid can be utilized as an oxidizing agent. In addition, when it is being heated, the acid loses sulfur and sulfur dioxide gas is released. Hot concentrated sulfuric acid reacts with most metals and also several nonmetals, such as sulfur and carbon. The concentrated sulfuric acid, due to its somewhat high boiling point, can release more unstable acids from their salts. An example of this reaction is when sodium chloride (NaCl, a common salt) is heated with concentrated sulfuric acid, hydrogen chloride (HCl) gas, appears. [4]
One of the most significant characteristics of concentrated sulfuric acid is its strong affinity for water (H2O). Therefore, the acid is frequently used to dehydrate many compounds as a drying agent. An example of this is carbohydrates. It also reacts with C12H22O11, sugar sucrose during which process sulfuric acid removes eleven molecules of water. The resultant compound is a fragile spongy black mass of carbon and diluted sulfuric acid. Similarly, sulfuric acid reacts with cellulose, skin, and even plants, etc. [4]
The dehydration process not only removes water from chemical compounds but also releases a legion of heat. The amount of heat released is actually adequate to even boil the water and spatter the acid. In order to produce diluted sulfuric acid, one must add cold water to the acid slowly with continuous stirring to hamper the buildup of heat. Then sulfuric acid chemically reacts with water. The resultant solution is called the diluted sulfuric acid, which are hydrates with different properties from pure or concentrated sulfuric acid. [4]
Video
General information on sulfuric acid - Periodic Table of Videos [26]
Reaction of sulfuric acid in sugar [27]
Gallery
References
- ↑ What is Sulfuric Acid? Conjecture Coporation, Shannon Kietzman, 10 Feb 2011.
- ↑ 2.0 2.1 2.2 2.3 History of Sulfuric Acid The Columbia Electronic Encyclopedia, 6th ed., 24 Jan. 2011.
- ↑ 3.0 3.1 3.2 Useful Sulphuric Acid Facts For Everybody Article Directory, 23 Jan. 2011
- ↑ 4.0 4.1 4.2 4.3 4.4 Concentrated Sulfuric Acid The Columbia Electronic Encyclopedia. Infoplease, 3 Feb. 2011.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 What is Acid Rain? United States Environmental Protection Agency, 8 Jun. 2007.
- ↑ Genesis 1 Biblegateway.com, 2 Dec. 2010.
- ↑ Cox, Porch, and Wetzel. Chemistry for Christian Schools. South Carolina: Bob Jones University Press, 2000. (p.537)
- ↑ Cox, p.529
- ↑ Cox, p.532
- ↑ Cox, p.531
- ↑ Sulfuric acid fccj.org, 4 Feb, 2011.
- ↑ Properties of Sulphuric Acid Ravensdown, 4 Feb, 2011.
- ↑ SULFURIC ACID, 52 - 100% Environmental Health & Safety, 4 Feb, 2011.
- ↑ Cox, p.530
- ↑ 15.0 15.1 15.2 15.3 15.4 Properties of Sulphuric Acid TutorVista.com, 4 Feb, 2011.
- ↑ 16.0 16.1 Sulphuric Acid (H2SO4) TutorVista.com, 4 Feb. 2011.
- ↑ 17.0 17.1 17.2 Sulfuric acid - uses dynamicscience.com 14, Feb. 2011.
- ↑ Sulfuric Acid Unknown, 24 Jan. 2011.
- ↑ History of sulfuric acid The Columbia Electronic Encyclopedia, 6th ed., 24 Jan. 2011.
- ↑ 20.0 20.1 Sulfuric Occurrences Pearson Education, Infoplease, 24 Jan. 2011.
- ↑ 21.0 21.1 21.2 Sulfuric acid uses The Columbia Electronic Encyclopedia, 6th ed, 23 Jan. 2011.
- ↑ Petroleum refinery The Encyclopedia of Earth, 19 Oct. 2006.
- ↑ 23.0 23.1 23.2 23.3 Acid Rain Virtual ChemBook, 3 Feb. 2011.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 24.6 24.7 24.8 Acid Rain ThinkQuest Education Foundation, 3 Feb. 2011.
- ↑ 25.0 25.1 Sulfuric Acid Facts Burgess, William A., Occupational and Environmental Epidemiology Branch, 12 Jan. 2011.
- ↑ Sulfuric Acid - Periodic Table of Videos periodicvideos, youtube.com, 1 July, 2010.
- ↑ Sulfuric acid in sugar angryewan, youtube.com, 25 Dec, 2008.
Additional Information
- [1] Sulfuric acid in sugar] angryewan, youtube.com, 25 Dec, 2008.
- [2] Sulfuric Acid - Periodic Table of Videos. periodicvideos, youtube.com, 1 July, 2010.
- [3] Sulfuric acid (wikipedia.com, 17 Feb. 2011.)
- [4] Bible (Biblegateway.com, 17 Feb.2011.)
- [5] Sulfuric acid (web.fccj.org, 17 Feb. 2011.)
- [6] Chemical of the weeki - Sulfuric acid (Chemical and Engineering News, June 25, 2001.)
- [7] Sulfuric acid (non Kietzman, 10 February 2011)
- [8] Sulfuric acid, 52 - 100%. MSDS Sheet (Environmental Health & Safety, 17 Feb. 2011.)
- [9] Sulfuric acid (chemicalland21.com, 17 Feb. 2011.)
| ||||||||||||||