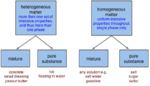
Mixture
A Mixture is a substance wherein there is a combination of two, three or more components. A mixture is differentiated from a compound, which is formed by the chemical combination of two or more pure substances in a fixed, definite equality between two ratios. The components of a mixture retain their own chemical properties and may be present in any equality between two ratios. Mixture is mixed in the form of solutions, suspensions, and colloids. Mixture can be classified into two sections: A homogeneous mixture a type of mixture in which the composition is uniform and every part of the solution has the same properties. A heterogeneous mixture a type of mixture in which the components can be seen, as there are two or more phases present in it. Mixtures can be in the food we eat, we see or even in our bodies as an example like blood. Mixtures are evident in our lives, and for the whole universe. Different types of mixtures can be used for creating new tastes, medicines and structures and other different types of things.That can be found in helping the human kind to grow economically and the nature in such as lands, foods, and animals who seek help.
Properties
When there is a combination of two, three or more substances it makes a mixture.The components of a mixture can be separated since the composition is not always constant. After separation and even during the mix of components, not all solution keep up their individual properties.Mixtures can be either homogeneous or heterogeneous. A homogeneous mixture is a type of mixture in which every part of the solution has the same properties. A heterogeneous mixture is a type of mixture in which the components are not evenly distributed throughout the mixture. [1]
A chemical mixture is a collection of molecules or atoms of different types. It's a sizably voluminous business of disseverment of mixtures, and disseverment science is a sub-division of chemistry. In a theoretical way mixtures are separable into their component elements or compounds by pristenly physical processes. [2]
Its quite unbelievable how mixture are almost everywhere we look. The whole nature considerably even in like rocks, the ocean or even the atmosphere are full of mostly mixtures. Mixtures are about physical properties, not chemical ones. If there is a change in chemical structure than it would be considered a reaction. There are a great number of mixtures everywhere, even in a simple food like cake. Just think about it, a cake is mixed with different ingredients, an so it becomes a mixture. Even a pencil is considered a mixture, the cellulose of the wood has a thousand other compounds and so is a mixture. There is way to tell a mixture, it's when you are capable of separating the substances in different physical ways. In a example like when you put sand in water it's considered a mixture, and than you can send it through a filter and once again out-put the sand. Mixtures can also be just both liquids id doesn't always require an . Like distilled water(H2O) full of water molecules and so is considered a pure substance, mixed with just oil would also be considered a mixture. [3]
Types of mixtures
There are 4 most known types of mixtures
Solution-In chemistry terms a solution Is considered a mixture-Substances dissolve in the other-It's a considered a mixture that is the same or in constant throughout. They are Homogenous mixtures. Example: Salt water Opposite: A heterogeneous mixture which is not a solution in constant throughout. Kind-Homogeneous mixture
Suspension-Between a liquid and particles of a solid, mixed throughout so that the particles are suspended throughout the whole liquid. Example: Mixture of water and sand- mixed and sand will be dispersed throughout the water. Kind: Heterogeneous
Colloid-Small particles of one substance are evenly distributed throughout another Solution- appear the same but are not, the particles are suspended in solution instead of dissolved. Example: Milk Kind: Heterogeneous Difference: In colloid the particles will stay floating/suspending instead of settling after a period time like in a suspension.
Alloy-Elements that have the characteristics of a metal and at least one of the elements mixed in are metal. Example: Steel-made of mixture of iron and carbon. kind: Homogeneous [4]
Interesting Facts
Mixtures can be separated into Solids, Liquids and Gases. Most substances we come in contact daily, even air, are considered mixtures. Saltwater is considered homogeneous while sand in water is considered a heterogeneous mixture. Smoke is considered a mix of particles that were suspended in the air. Even our blood is considered a mixture which between it's two main parts plasma and red blood cells can be separated by a machine called centrifuge. While in a homogeneous mixture all substances are evenly distributed, in a heterogeneous mixture the substances are not evenly distributed. [5]
Mixtures can include either at least two different types of atoms or of molecules, or at least one type of atom with at least one type of molecule. Mixtures can be separated back into their individual substances by using physical ways. Many times the components of a mixture are evidently seen, like an example you can see the grains of sand in cup of water. For many cases using a magnet to separate iron filings, from a mixture of iron filings (dark grey color) and sulfur (yellow color) can help separate a mixture much more easily.[6]
Chemistry is classified as the study of physical matter in many unique ways. Some of it's ways that its classified are the state of matter (gas, liquid or solid), chemical form (element, mixture or compound), chemical structure (atoms or molecules, etc.) and so on. [7]
Video
Type of mixtures. Three types of mixtures: suspensions, colloids, and solutions.
References
- ↑ Chemical Mixtures Ducksters. Web. last updated May, 2015. Unknown Author.
- ↑ Chemical Mixtures Jrank. Web. Accessed 19, 2015. Unknown Author.
- ↑ Mixture Basics Rader's Chem4kids. Web. Accessed 19, 2015. Unknown Author.
- ↑ Chemical Mixtures Ducksters. Web. Last updates May, 2015. Author Unknown.
- ↑ Chemical Mixtures Ducksters. Web. Last updated May, 2015. Author Unknown.
- ↑ What is a Mixture... in terms of Chemistry? IvyRose Holistic. Web. Accessed May 19, 2015. Author Unknown.
- ↑ Elements, Mixtures, and Compounds IvyRose Holistic. Web. Accessed May 19, 2015. Author Unknown.
| ||||||||||||||