Sodium bicarbonate
| Sodium bicarbonate | |
|---|---|
| |
| General | |
| Systematic name | Sodium Hydrogen Carbonate |
| Other names |
Sodium hydrogen carbonate |
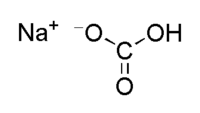
| Molecular formula | NaHCO3 |
| SMILES | C(O)(=O)[O-].[Na+] |
| Molar mass | Molar mass::84.01 |
| Appearance | White, crystalline powder |
| CAS number | CAS number::144-55-8 |
| Properties | |
| Density and phase | [[Density:: 2.16 g/cm-3 solid]] |
| Solubility in water | 7.8g/100g water (18°C) |
| Melting point | Melting point::270°C |
| Decomposition | 50°C (loses CO2) |
| Acidity (pKa) | HCO3- <-> CO32- + H+ pKa = 10.33 CO2 + H2O <-> HCO3- + H+ pKa = 6.33 |
| Structure | |
| Molecular shape | Trigonal Planar |
| Crystal structure | Monoclinic-prismatic |
| Hazards | |
| MSDS | Material Safety Data Sheet |
| Main hazards | Slightly hazardous if ingested or inhaled;
mild irritant to eyes and skin. |
| NFPA 704 | |
| Flash point | Not applicable |
| RTECS number | VZ0950000 |
| Related compounds | |
| Other anions | Sodium carbonate |
| Other cations | Sodium carbonic acid |
| Related compounds |
CH3NaO3 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Sodium bicarbonate (NaHCO3), recognized by most as ordinary baking soda, is a compound known for its unusual properties and diverse applications. Found in deposits throughout the globe, natural sodium bicarbonate works to maintain the Earth's balance of carbon dioxide and pH. [1] Baking soda was first used mainly for cooking purposes, but over the last 150 years, its production has grown to include manufacturing of other metals and glass, and as a basic household cleaner. This natural substance has also increased in popularity because it has no adverse effects on the environment.
Properties
Sodium bicarbonate (most commonly known as baking soda) is classified as an acid salt. This classification results from the combination of the base sodium hydroxide and the acid carbonic, which also gives baking soda the properties of a mild alkali. When heated, sodium bicarbonate releases carbon dioxide and it reacts as a leavening agent for culinary purposes as well as a fire retardant. Carbon dioxide is heavier than the air fueling the fire, so the baking soda smothers the flames. Sodium bicarbonate is capable of precipitating calcium and can absorb acid gas emissions such as sulfur dioxide. [2] Baking soda exists as a solid composed of white, monoclinic crystals and weighs 84.01 g/mol. [3] Because sodium bicarbonate begins to decompose at 50°C, it is difficult to properly determine a boiling or melting point. [4] Its decomposition produces sodium carbonate (Na2CO3), carbon dioxide (CO2), and water (H2O). [5] Baking soda is neither flammable nor explosive. Its chemical attributes also prevent oxidization and make it insoluble (doesn't dissolve) in alcohol. Although it is a mild irritant to the eyes, baking soda remains otherwise non-toxic and safe for general use. [6]
Occurrences
Most sodium bicarbonate is produced through artificial means, but in nature it's found in a mineral called nahcolite. The name Nahcolite is a mnemonic for its chemical formula (Na+H+CO+lite). This mineral, known as an evaporite (“A sedimentary deposit that results from the evaporation of seawater”) [7] , forms in oil shales and lake sediment deposits, and capable of absorbing water. Nahcolite coloring ranges from colorless to brown, and the crystals are monoclinic and transparent. Nahcolite has been found most notably in California, Colorado, Egypt and Kenya. Although the first major find of nahcolite was near Searles Lake, California in 1940 [8] , it was first reported by P. Walther in East Africa. In the oil-shale riverbed of the Green River (Colorado), nahcolite deposits as large as five feet in diameter and other formations with layers four inches thick, have been discovered. Sodium bicarbonate is rarely found in nature because, when exposed, it loses carbon dioxide and passes into another mineral called trona (hydrated sodium bicarbonate carbonate). When water vapor and carbon dioxide react with trona, sodium bicarbonate is a resulting byproduct. Nahcolite only forms when there is excessive and contained carbon dioxide; otherwise, trona will form in its place. [9]
Prior to the late 1800s, the Leblanc process was used to manufacture soda ash (sodium carbonate), from which sodium bicarbonate is produced. This process required the creation of sodium sulfate by heating up sodium chloride (table salt) and sulfuric acid. In the late 1800s however, this process was abandoned in favor of the Solvay method in which ammonium and carbon dioxide are exposed to a concentrated solution of sodium chloride. This process creates crude sodium bicarbonate that is refined into pure baking soda. Before being used in food products or for pharmaceutical purposes, the refined baking soda must pass the specifications laid out by the U.S. Food and Drug Administration (FDA) and the United States Pharmacopoeia and Food Chemicals Codex. This ensures the quality and safety of the finished product. [10]
Uses
Although many people associate baking soda with its role as a cleaner and deodorizer, its chief use is in the food industry. In the United States alone, 33% of baking soda production is consumed in this manner. When used in baking as a key ingredient in baking powder, sodium bicarbonate reacts and releases carbon dioxide. This makes it the most commonly used leavening agent in cooking. Carbonated soda manufacturers have taken advantage of this reaction and frequently add baking soda to drinks to create carbon dioxide bubbles. Much of sodium bicarbonate production goes toward feed supplements for animals, accounting for approximately 25% of its use. For poultry, this valuable supplement enables them to tolerate heat, increases the quality of the eggshell, and keeps up the balance of sodium. Cows need sodium bicarbonate to properly digest fiber and sustain the pH of the rumen. Chemists utilize baking soda as a catalyst, buffer, a blowing agent, and feedstock for chemicals. Other industries that use sodium bicarbonate include pharmaceuticals- as a buffering agent, source of carbon dioxide, and an antacid; leather tanning (maintains pH during the tanning of hides); and the production of sodium carbonate. Because of its weak basicity, baking soda counteracts the effects of nasty smells or odors, and can even act as a fire suppressant. (Myers 248) [11] Baking soda is also employed in the production of glass, laundry detergents, soaps, paper, as well as aluminum refining and other processes of manufacturing various metals. [12]
Baking soda is a popular chemical compound for household purposes because of its versatility, safety, and availability. Its existence as a natural chemical makes it environmentally safe to use and it can even be recycled around the house. [13] The applications of baking soda for everyday use are endless, including, but not limited to: toothpaste, skin irritations (cuts, allergies, etc.), general household cleaner, refrigerator deodorizer, canning, pest and rodent repellant, and stain remover. (Summary of Hughes 1-41) [14]
Household Antacid
One of the most popular and common applications of sodium bicarbonate is a homemade remedy for heartburn. Known as an antacid (a compound that absorbs and neutralizes stomach acid), simple baking soda can temporarily relieve symptoms of indigestion and occasional heartburn. When taken with food, the effects of sodium bicarbonate can last for two to three hours, but at best it is something to be used infrequently and is not a replacement for prescription medication. [15] Heartburn happens when people eat too rapidly or eat certain foods, which causes a small amount of stomach acid to enter the esophagus and produce a burning feeling in the chest. Baking soda can neutralize the acid because of its alkaline properties, and is often used because of its availability and affordability. If a person overdoses on sodium bicarbonate however, it can neutralize too much of the stomach's acid and prevents the proper digestion of food. This can lead to inflammation and allergies throughout the body. [16] Baking soda is not a suitable medication for people suffering from edema, high blood pressure, or heart disease because it increases the level of sodium in the body which aggravates these health conditions. Sodium bicarbonate may also interfere with the effectiveness of other medications like iron supplements or aspirin. If sodium bicarbonate is only administered occasionally and other medications are used an hour or more later, there is rarely any trouble. Overall, baking soda as an antacid provides immediate and temporary relief to heartburn and indigestion, but should not be used in the case of daily symptoms which could indicate a more serious health condition. [17]
References
- Arm Hammer Bicarbonate of Soda Jackson Siegelbaum Gastroenterology (gicare.com)
- Heartburn, Baking Soda and Antacids - What You Must Know Beech, Natalie. Body for Mind.
- Sodium Bicarbonate National Center for Biotechnology Information. PubChem.
- [http://www.chem.unep.ch/irptc/sids/OECDSIDS/Sodium%20bicarbonate.pdf SODIUM BICARBONATE
CAS N°: 144-55-8] Organisation for Economic Co-operation and Development (OECD). Screening Information Data Set (SIDS).
- The 100 Most Important Chemical Compounds- A Reference Guide Richard L. Myers.
- Baking Soda Book- Resourceful and Ingenious Uses of Baking Soda Hughes, George (Tipking).
- Baking Soda How Products are Made.
- SODIUM BICARBONATE (NAHCOLITE) FROM COLORADO OIL SHALE Tell Ertl. Bureau of Mines.
- The Mineral Nahcolite Amethyst Galleries.
| ||||||||||||||




