Sodium chloride
| Sodium chloride | |
|---|---|
 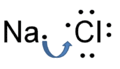
| |
| General | |
| Systematic name | Sodium chloride |
| Other names |
Common salt |
| Molecular formula | NaCl |
| Molar mass | Molar mass::58.44 g/mol |
| Appearance | Colorless crystals |
| CAS number | CAS number::7647-14-5 |
| Properties | |
| Density and phase | Density::2.165 g/ml, solid |
| Solubility in water | 35.9 g/100 ml (25°C) |
| Melting point | Melting point::801°C |
| Boiling point | Boiling point::1,413°C |
| Structure | |
| Coordination geometry |
Octahedral (Na+) |
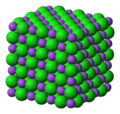
| Crystal structure | Face-centered cubic |
| Hazards | |
| MSDS | Material safety data sheet |
| Main hazards | Irritant and may sting |
| NFPA 704 | |
| Flash point | Non-flammable |
| R/S statement | R: None S: None |
| RTECS number | VZ4725000 |
| Related compounds | |
| Other anions |
Sodium fluoride |
| Other cations |
Lithium chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Sodium chloride is one of the most well known compounds. The chemical formula is NaCl, and it is an ionic compound. Sodium chloride is solid and it is soluble in water. Sodium chloride is one of the most unique compound, because it is not only found in nature but also can be created by chemical response at the laboratory. Sodium chloride is very useful and important. Because it is used for flavoring and prevention of food turns bad. It is important for the food industry. Sodium chloride was used as money and a symbol of wealth in the past. Besides this, sodium chloride can be used as part of a material to make glass or pulp industry. In conclusion, sodium chloride is very essential compound for mankind. Also, sodium chloride is important for human and animal bodies. It helps control our body's fluid balance and helps send nerve impulses. However, excessive amounts of sodium chloride is harmful to our body. Therefore, people need to reduce and control the intake of sodium chloride.
Properties
Sodium chloride is one of the most well known and widely used chemicals which known as table salt. Sodium chloride is odorless and it has a distinctive taste. The properties of Sodium chloride can divided into two parts, physical properties and chemical properties. Sodium chloride is small, transparent, and made of colorless white cubic crystals. Also it is forms powder or granules. The chemical formula of sodium chloride is NaCl and the molar mass is 58.44 g/mol. It is an ionic compound which consists of a sodium cation Na+ and a chloride anion Cl-. Solid Sodium chloride has a crystalline structure from each Na+ ion and it is surrounded by six chloride ions in an octahedral structure. The density of Sodium chloride is 2.16 g/mL and a melting point is 801 °C. Sodium chloride is available as aqueous solutions of different concentrations called saline solutions (A solution of sodium chloride in water[1]).[2]
Sodium chloride is readily soluble in water and other polar solvents. And it is insoluble or only slightly soluble in most other liquids.[3] This is because Sodium chloride is a stable solid. It only decomposes at high temperatures to give toxic fumes of hydrochloric acid and disodium oxide.[2] Sodium chloride is an ionic compound which is made up of equal numbers of positively charged sodium and negatively charged chloride ions. Because of the Sodium chloride is melted or dissolved in water and the ions can move freely, dissolved or molten sodium chloride is a conductor of electricity and it can be decomposed into sodium and chlorine by passing an electrical current through it.[3]
Occurrences and Synthesis
-Occurrences-
Sodium chloride is found as a compound in nature with high reactivity.[4] It is present in 80% of the dissolved material in the seawater. About 1 to 5 percent of seawater is made of sodium chloride.[2] 1 gallon of seawater contains 0.231 pound of salt. Usually salt is obtained by evaporation of shallow seawater caused by the sun in the warm climates.[5] Sodium chloride is also found as the mineral halite, which also called a rock salt. Halite can be found in underground deposits. Those deposits are usually find in mine and sometime, water is pumped down the brine.[6]
Underground deposits formed when ancient oceans evaporated. When the earth movements buried the deposits, they mined to remove the sodium chloride.[7] Halite is containing about 25 percent of sodium chloride on the surface. The brine is evaporated by vacuum evaporators. Therefore, impurities separate first and can be removed.[6]
-synthesis-
According to the synthesis, the way to make sodium chloride is to react the hydroxide with hydrochloric acid. The salt can be purified by recrystallization, and sodium metal reacts actively with all the halogens to form sodium halides. So, it burns with chlorine and that form sodium chloride.[8]
Uses
Sodium chloride, which called table salt is very important in our life. Sodium chloride has great importance to human and animal health. It is a part of most animal fluids, such as blood, sweat, and tears. Also it is an essential part of the diet of both humans and animals. It helps digestion by providing chlorine for hydrochloric acid, which is a small but essential part of human digestive fluid.[3] Sodium chloride use widely in the food industry for flavoring and preservation. It has been used as the meat packing, fish curing, and preservative or seasoning for food.[5] Sodium chloride is used in the production of many important chemicals like sodium hydroxide, sodium carbonate, baking soda, and hydrochloric acid. Also it is a part of a material to make glass, soup, glaze, pottery, textile dyes, oil refineries, paper and pulp industry, fire retardants, and rubber industry. Another important use of sodium chloride is the de-icing of roads and sidewalks in cold and snowy season. When the Sodium chloride go on the snow or ice, salt make the melting point of the mixture lower. Therefore, large amounts are used in northern climates to help rid snow and ice on the on streets and highways. [2]
Historically, Salt was used in some parts of the Western Hemisphere and in India. The use of salt was introduced by Europeans. However, salt was a luxury available only to the rich in parts of central Africa. Later, salt have been used as money in Ethiopia and elsewhere in Africa and in Tibet.[5] However, in the France, a high tax on salt was a contributing cause of the French Revolution.[3] Today, China, the United States, India, Germany, Canada, and Australia are the world’s largest salt producers in the early 21st century.[5]
Health effects (Biological role)
Sodium chloride is essential for our life.[9] sodium chloride is non-toxic and non-hazardous, and is an important electrolyte (A substance that produces an electrically conducting solution when dissolved in a polar solvent, such as water.[10]) source for the body.[2] Therefore, Sodium chloride has a lot of important functions in plants, humans, and animals. In human body, sodium chloride helps control our body’s fluid balance.[7] The sodium chloride allows body to retain water and helping to maintain the volume of fluid in the body. Having enough fluid ensures protects against low blood pressure.[11] Sodium chloride also helps send nerve impulses and affects muscle function.[7] Sodium supports proper nerve functioning by playing a role in action potentials. During an action potential, sodium rushes out of nerve cells to initiate the electrochemical impulse.[11] If people do not have enough sodium in their body, it takes medication, such as serum sodium, in order to maintain a healthy amount of sodium in the body.[4]
Sodium chloride is essential for our life. However, there are many negative health effects by excess of sodium chloride. Kidneys have trouble by keeping up with the excess of sodium chloride in the bloodstream. When sodium accumulates, the body holds onto water to dilute the sodium. Therefore, the volume of blood is increase in the bloodstream. Also, too much salt can damage the heart, aorta, and bones. [12] Sodium chloride can cause symptoms like vomiting, nausea, osteoporosis, diarrhea and dehydration. It also irritates the eyes and causes eye damage at high concentrations.[2] To prevent these symptoms, if someone is younger than age 51, they should consume no more than 2,300 mg of salt per day. And if someone is older than age 51, they should consume no more than 1,500 mg of salt per day.[13]
Video
This video is process of how to make sodium chloride
References
- ↑ Saline Wikipedia. Web. Accessed October 6, 2016 unknown author
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Sodium chloride Softschools.com. Web. Accessed November 1, 2016 unknown author
- ↑ 3.0 3.1 3.2 3.3 Sodium chloride Infoplease. Web. Accessed November 1, 2016 unknown author
- ↑ 4.0 4.1 Chemistry of sodium Libertexts. Web. November 12, 2016 unknown author
- ↑ 5.0 5.1 5.2 5.3 Hills, John. Sodium chloride Encyclopadia Britannica. Web. August 23, 2016
- ↑ 6.0 6.1 Sodium chloride yuvaneeds.com. Web. November 12, 2016 unknown author
- ↑ 7.0 7.1 7.2 Sodium chemistryexplained. Web. November 12, 2016 unknown author
- ↑ Sodium:Sodium chloride WebElements. Web. November 12, 2016 unknown author
- ↑ Sodium and your health Breakupwithsalt. Web. November 13, 2016 unknown author
- ↑ https://en.wikipedia.org/wiki/Electrolyte Electrolyte] Wikipedia. Web. Accessed November 4, 2016 unknown author
- ↑ 11.0 11.1 What Role Does Sodium Play Biologically? sfgate. Web. November 13, 2016 unknown author
- ↑ Health Risks and Disease Related to Salt and Sodium Harvard T.H. Chan. Web. November 13, 2016 unknown author
- ↑ The Effects of Sodium Chloride on Your Body livestrong.com. Web. November 13, 2016 unknown author
| ||||||||||||||





