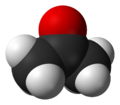
Acetone
| Acetone | |
|---|---|
 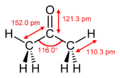
| |
| General | |
| IUPAC name | Propanone |
| Other names |
DMK |
| Molecular formula | C3H6O |
| SMILES | CC(=O)C |
| Molar mass | Molar mass::58.08 g mol−1 |
| Appearance | Colorless liquid |
| CAS number | CAS number::67-64-1 |
| Properties | |
| Density and phase | Density::0.79 g/cm3 |
| Solubility in water | miscible |
| Melting point | Melting point::−94.9 °C |
| Boiling point | Boiling point::56.53 °C |
| Viscosity | 0.32cp at 20°C |
| Structure | |
| Molecular shape | trigonal planar at C=O |
| Dipole moment | 2.91 DD |
| Hazards | |
| MSDS | Material safety data sheet |
| NFPA 704 | |
| Flash point | -17 °C |
| R/S statement | R: R11, R36, R66, R67 S: (S2), S9, S16, S26 |
| RTECS number | AL31500000 |
| Related compounds | |
| Related sovents | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Acetone is an organic solvent of industrial and chemical significance. Although acetone is aromatic and flammable at 56.2°C, it is very colorful.[1] OC(CH3)2 is the formula of acetone. Acetone is a very common thing to use in the household such as paint thinner and sanitary cleaner/ nail polish remover base. It is produced naturally and the human body also produces some of acetone by itself.[2]
Properties
Ketones is the common name for acetone. CH3COCH3 is the correct formula of acetone. The IUPAC, which stands for the International Union of Pure and Applied chemistry, sets acetone as 2-propane and dimethyl ketone. In the several ways, acetone can be produced from petrochemical sources. During those methods. It takes oxidation of 2-proposal, hydration of propene, and a co-product of the O2-oxidation of cumene.[3]
Occurrences
Acetone occurs naturally in many type of ways. It is produced by trees, volcanic gases, forest fires, and a product of the breakdown of body fat. Most acetone is produced in the industrial company and it dffects the environment badly.[4] About 97% of acetone is produced by manufacturers, and it moves into the air and it becomes rain or snow.[5] Propane helps to produce acetone. Cumene which is an organic compound and also is called isopropylbenzene,it is produced by benzene and it also effects phenol and acetone.[6]
Uses
Acetone is used as a solvent that is formed with plastics and synthetic fibers. It helps to clean fiberglass tools and make fiberglass resin thinner. Before two part, epoxies and superglue become hard one, they can be dissolved by acetone. Also, many plastics dissolved by aceton. To make Nalgene bottles which made of polystyrene, it needs acetone. Also, it is used to paint the preparatron of metal. About millions of kilograms of acetone are produced as the solvents such as methyl isobutyl alchol and methyl isobutyl ketone. Acetone is also used in the pharmaceutical industry and it is part of pharmaceutical products.[7] To make plastic, fibers, drugs, and other chemicals, acetone is necessaries.[8] Acetone is also used to protect skin contact and maintain eye wash fountain.[9] Acetone also can be used as nail polish remover. Ethyl acetate, and another solvent are very similar as acetone, they also are used as solvent. Acetone is excellence to easily remove residues and manicures from a nail.
Safety
Acetone is flammable at a temperature of 465°C,it making dangerous. When a temperature goes over acetone's flash point then air mixes with 2.5% and 12.8% acetone. Also, vapor from acetone causes to fire. Acetone peroxide is a highly unstable compound that it is produced when it oxidizes. It makes friction and shock sensitives.[10] If our body het acetone then it enters our blood and it takes all organs from the body. This happening is harmful to the human body even if small amount of organ were stealed because the liver can't work well after that. It also effects our breathing systems like nose, throat, lung, and eye irritation. This cause headache, confusion, or increaing pulse rate.[11] If people eat large amounts of acetone then it gives very harmful damage to the mouth and also affects the skin if they come in contact with it. So, people should treat this chemical products carefully.[12] Acetone doesn't cause the cancer that the department of health and human service couldn't find any production of cancer on acetone in their examination. But it may not precision so they do not sure of that acenton may cause the cancer.[13]
References
- acentone jtbaker, unknown authors
- safety of acetone ATSDR
- acetone multiple authors
- properties BookRags, unknown authors
| ||||||||||||||



