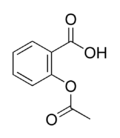
Aspirin
| Aspirin | |
|---|---|
| |
| General | |
| Systematic name | 2-(acetyloxy)benzoic acid |
| Other names |
Salicylic acid acetate |
| Molecular formula | C9H8O4 |
| SMILES | CC(=O)Oc1ccccc1C(=O)O |
| Molar mass | Molar mass::180.15 |
| Appearance | Transparent, colorless crystals. |
| CAS number | CAS number::50-78-2 |
| Properties | |
| Density and phase | [[Density::1.4 g/cm3]] solid |
| Solubility in water | 1g/100 ml (37°C) |
| Melting point | Melting point::135°C (with decomposition) |
| Boiling point | Boiling point::140°C |
| Hazards | |
| MSDS | External MSDS sheet |
| Main hazards | toxic is swallowed in large quantity |
| NFPA 704 | |
| RTECS number | VO0700000 |
| Related compounds | |
| Related compounds | Salicylic acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Aspirin is an organic compound containing nine carbon atoms, eight hydrogen atoms, and four oxygen atoms. Apsirin has been used different cultures and people groups throughout history. Hippocrates used a form of the medicine in ancient Greece, but it was not until the 1830s that it was figured out that it was salicylic acid. Just recently aspirin has been equated with helping people with heart problems and it has been suggeseted that most people take one a day.
Properties
Aspirin is a colorless, odorless crystal. It maintains stability when in dry conditions, but when introduced to moisture it begins to decompose into acetic acid and salicylic acids [1].
Occurrences
Acetylsalicyclic acid is a derivative of salicylic acid [2]. Salicylic acid is found in plants like wintergreen leaves, birch bark, and as well the bark and leaves of the willow tree. It can also be produced by adding sodium phenolyate and carbon dioxide with heat [3]. The salicylic acid alone was bitter though, so it was synthesized with sodium.
Uses
Aspirin is best known for its medicinal value. Aspirin a non prescription, nonsteroidal anti-inflammatory drug. It is used for treating pain, fevers, and swelling associated with arthritis, cramps, tendinitis, and other injuries. It is a fast relief for mild and moderate pain [4].
Nonsteroidal anit-inflamatory drugs (NSAIDs) inhibit the enzyme cyclooxygenase and their production of prostaglandins. Prostaglandins protect the stomach lining and promote pain, inflammation, fever, and blood clotting. NSAIDs usually inhibit blood clotting for a few hours, but Aspirin, specifically stops blood clotting for an extended period of time, between four and seven days. For this reason Aspirin makes a great drug for the prevention of heart problems from blood clots [5]. Aspirin does have its risks though. Because of its great prevention of blood clots it carries the risks of stomach ulcerations and bleeding [6].
Aspirin comes in can be taken orally, but also rectally. It comes in either chewable tablets or enteric coated tablets or caplets. [7]. 81 mg, low dosage tablets, are available for daily use helpful for maintaining normal platelet aggression and the prevention of heart problems [8]. Normal strength 325 mg tablets are available as well as extra strength 500 mg tablets. The drug should be taken with food to prevent an upset stomach and absolutely not with alcohol. Even heavy drinkers (those who consume more than three drinks a day) should talk to their doctors before taking aspirin[9].
Aspirin should not be taken by everyone and you should talk to your doctor before taking it regularly. Children and teens should not take aspirin with flu like symptoms or the chicken pox because of the risk of Reye syndrome [10]. Pregnant and nursing mothers should also talk to their doctor because Aspirin is excreted in the breast milk [11].
The History of Aspirin
Aspirin is derived from a drug found in willow trees, salicylic acid. Hippocrates himself left records of the ancient Greeks using the barks and leaves of the willow tree for help fighting fevers and pain[12]. In the 1830s it was discovered that it was the salicin compound in the willow plant that gave pain relief. In 1832 a German scientist took this information and started experimenting with salicin; he created salicylic acid. The creation of salicylic acid was a success, but wash harsh on stomaches. In 1853 Charles Frederic Gerhardt, a French chemist, was able to eutralize the compound and created acetylsalicylic acid [13]. In the year 1897 a chemist from Bayer in Germany, Felix Hoffmann, successfully synthesized a stable form of acetylsalicylic acid that he gave to his father to provide pain relief of his rheumatism. Soon after Hoffman introduced his new powdered drug to his patients and convinced Bayer to sell the product [14] [15]. In 1915 tablets were created and the drug became non prescription. In 1919 Bayer had to give up its trademark to Aspirin as a result of Treaty of Versailles. In 1948 a California practitioner noticed the positive affects of Aspirin and started to recommend "An Aspirin a day". It wasn't until the beginning of the 1970s that scientists were able to discover how exactly aspirin worked and that it blocked prostaglandins. Throughout the 1980s and 1990s doctors began to realize how useful aspirin was. In 1998 its prevention of cardiovascular problems was proven if taken in low doses [16].
References
- ASPIRIN Environmental Health & Safety. 01/23/07.
- Acetylsalicylic acid (Aspirin, Ecotrin) Medicinenet.com. 12/31/1997.
- Nonsteroidal Antiinflamatory Drugs (NSAIDs) by Omudhome Ogbru, Pharm.D. Edited by Jay Marks, M.D. MedicineNet.com
- Low Dose Aspirin Life Extension Foundation. © 1995-2009.
- An Aspirin a Day by Tamar Nordenberg. U.S. Food and Drug Administration.FDA Consumer magazine. March-April 1999.
- History of Aspirin About.com.
- Aspirin History Bayer Healthcare. Copyright ©2008.
- How to Make Aspirin - Acetylsalicylic Acid By Anne Marie Helmenstine, Ph.D. About.com
- How does aspirin crystallize? Angewandte Chemie International Edition 2007, 46, No. 4, 615–617, doi: 10.1002/anie.200602378.
| ||||||||||||||




