Elétron
Elétrons são partículas carregadas negativamente que formam um componente principal da matéria. Toda a matéria é constituída por átomos, e os átomos consistem de componentes eletricamente carregados - os elétrons negativos e leves, e o núcleo positivo.
A palavra "elektron" em Grego significa âmbar, resina fossilizada amarela de árvores perenes, um "material plástico natural" já conhecido pelos gregos antigos. Era conhecido que, quando o âmbar era esfregado com pano seco--produzindo o que agora se chamaria eletricidade estática--poderia atrair objetos leves, como pedaços de papel.[1]
História

Durante os anos 1800, tornou-se evidente que a carga elétrica tinha uma unidade natural, que não poderia ser subdividida mais longe, e em 1891 Johnstone Stoney propôs nomeá-la "elétron." Os experimentos, como o "efeito Edison" descoberto pelo inventor dos EUA Thomas Alva Edison (1847-1931),foram importantes na determinação da massa das partículas emitidas, que ficaram conhecidas como "elétrons". O experimento do "efeito Edison" envolveu um bulbo de vidro para com bombeamento de saída, em que um circuito elétrico é completado por elétrons emitidos a partir de um fio quente. Edison patenteou o fenômeno em 1883 e muitos dispositivos eletrônicos usam hoje em dia. Descobriu-se que eles eram bastante leves. The simplest atom, that of hydrogen, contains a central positive particle, a proton, and a single electron, and the proton is nearly 2000 times heavier.[1]
Experiments with beams of negative particles were performed in Britain by Joseph John ("J.J.") Thomson, and led to his conclusion in 1897 that they consisted of lightweight particles with a negative electric charge, nowadays known as electrons. Thomson was awarded the 1906 Nobel Prize. William Gilbert, a physician who lived in London at the time of Queen Elizabeth I and Shakespeare, studied magnetic phenomena and demonstrated that the Earth itself was a huge magnet, by means of his "terrella" experiment. But he also studied the attraction produced when materials such as amber were rubbed, and named it the "electric" attraction. From that came the word "electricity" and all others derived from it. When J.J. Thomson discovered the light particle which carried that charge, the name "electron" was applied to it. The many applications of electrons moving in a near-vacuum or inside semiconductors were later dubbed "electronics."[1]
Atomic configuration
Shells
The electrons of atoms orbit at varying distances from the nucleus, which are known as shells or energy levels. Within each shell reside one or more distinct electron orbitals. The electron shells and the orbits they contain make up the electron configuration of an atom. (see: Gallery of electron shells for elements.)
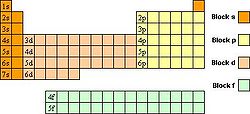
The configuration of electrons primarily determines an elements chemical reactivity, and particularly the outer-shell (or "valence") electrons. Because of this, the elements in the periodic table are arranged into rows (known as groups) based on how many shells of electrons an atom possesses, and into columns (known as periods) consistent with the number of electron in outer-shell.
Because of the importance of the outermost shell, the different regions of the periodic table are sometimes referred to as periodic table blocks, named according to the sub-shell in which the "last" electron resides, e.g. the s-block, the p-block, the d-block, etc. See image at right.
Orbitals
An electron shell is a group of atomic orbitals with a principal quantum number (n) of the same value. The maximum number of electrons that can be held in each shell can be calculated using the formula 2n2 where n is the shell number. Therefore, the first shell can hold only 2 electrons, the second 8, then 18, 25, 32, and 50.
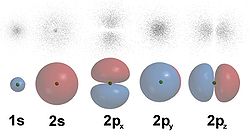
Electron shells are made up of one or more electron subshells, or sublevels, which have two or more orbitals with the same angular momentum quantum number (l). These orbitals are identified by the letters s, p, d, f, g, h, i, etc. Each orbital can hold a maximum of 2 electons and is distinguished by its unique shape. For example, S orbitals are circular, whereas P orbitals are dumbell shaped.
These subshells correspond to the azimuthal quantum numbers (l-values) 0, 1, 2, 3, 4, 5, 6, etc. Each shell can hold up to 2, 6, 10, 14, 18, 22 and 26 electrons respectively, or 2(2l + 1) electrons in each subshell.
Notation

In the ground state of an atom (the condition in which it is ordinarily found), electrons are found to occupy the lowest-energy level possible, and enter into higher levels in order of increasing energy. For example, the first electron goes into the lowest level, the second into the next lowest, and so on. This is known as the Aufbau principle.
Chemists use a standard notation to describe atomic electron configurations. In this notation, a subshell is written in the form nxe, where n is the shell number, x is the subshell label and e is the number of electrons in the subshell. The periodic table at right illustrates the notation given to each element. These notations assume knowledge of the electron configuration in the lower energy levels.
Referências
- ↑ 1,0 1,1 1,2 History of the Electron by Dr. David P. Stern, "The Exploration of the Earth's Magnetosphere". National Aeronautics and Space Administration. Última atualização 25 de novembro de 2001.
| ||||||||||||||