Trinitrotoluene
| Trinitrotoluene | |
|---|---|
| |
| General | |
| Systematic name | 2-Methyl-1,3,5-trinitrobenzene |
| Other names | 2,4,6-Trinitrotoluene, TNT, Trilite, Tolite, Trinol, Trotyl, Tritolo, Tritolol, Triton, Tritone, Trotol, Trinitrotoluol, 2,4,6-Trinitromethylbenzene |
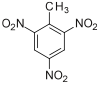
| Molecular formula | C7H5N3O6 |
| SMILES | O=[N+]([O-])c1c(c(ccc1C) [N+]([O-])=O)[N+]([O-])=O |
| Molar mass | Molar mass::227.13 g/mol |
| Appearance | Pale yellow solid |
| CAS number | CAS number::118-96-7 |
| Properties | |
| Density and phase | Density::1.654 g/cm3, Solid |
| Solubility in water | 0.13 g/L (20 °C) |
| Other solvents | Ether, Acetone, Benzene, Pyridine |
| Melting point | Melting point::80.35°C (176.63 °F; 353.50 K) |
| Boiling point | Boiling point::240.0 °C (464.0 °F; 513.1 K) (decomposes) |
| Structure | |
| Dipole moment | 1.37 D |
| Hazards | |
| MSDS | ICSC 0967 |
| Main hazards | Explosive (E) Toxic (T) Dangerous for the environment (N) |
| NFPA 704 | |
| Flash point | 167 °C (333 °F; 440 K) |
| R/S statement | R: R2, R23/24/25, R33, R51/53 S: (S1/2), S35, S45, S61 |
| RTECS number | XU0175000 |
| Related compounds | |
| Related compounds | picric acid hexanitrobenzene 2,4-Dinitrotoluene |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Trinitrotoluene is a chemical compound commonly known as TNT. TNT is commonly confused with dynamite, however, dynamite is actually a mixture of nitroglycerin and an absorbent agent such as sawdust.[1] TNT is characterized by its pale, yellow color. It is extremely explosive, but also relatively stable. It is not produced commercially within the United States, but can be found in military arsenals.
Properties
Physical Properties
Trinitrotoluene (TNT) is a pale yellow [2] crystalline solid at room temperature[3]. It is completely odorless and can exist as a solid or in crushed flakes. TNT is known to be soluble in water (111mg/L at 23 °C) and has a boiling point of 240 °C (464 °F), at which temperature it explodes. It has a melting point of 80.1 °C (176 °F). TNT has a density of 1.65 g/cm3 and a vapor density of 7.85 (Air = 1).[2] Trinitrotoluene has a molecular weight of 227 g/mol.[4]
Chemical Properties Trinitrotoluene is recognized as a toxic substance to humans. It is stable, [1] it does not react to friction or shock, but is highly explosive with a proper detonator.[3] Trinitrotoluene is nonreactive enough to be poured in molten liquid form into casings[1], yet it reacts violently, with the risk of an explosion, with reducing agents, and also reacts with heavy metals.[5] There are several substances that TNT is incompatible with. All strong Oxidizers, Ammonia, strong alkalies,[4] combustible materials, and heat are incongruous with Trinitrotoluene. Rapid heating is even known to cause explosions.[5]
Synthesis / Occurrences
The production of Trinitrotoluene is a process of three steps.[1] It involves nitrating toluene with nitric acid and sulfuric acid. The basic procedure is to increase temperature and mixed-acid concentrations to cause nitro groups to form mononitrotoluene, dinitrotoluene, and trinitrotoluene. These steps can be completed separately or in a continuous process.[6] The first step is to mix the nitrated toluene with the nitric and sulfuric acids, which produces the mononitrotoluene.
The next step is to remove the mononitrotoluene and nitrate again, after which dinitrotoluene is formed. The final step is to nitrate one more, and trinitrotoluene is produced.[1] An aqueous sodium sulfite solution is used[6] to remove isomers, which make the TNT more unstable.[1] Several other compounds are also formed throughout the process. TNT is not produced commercially in the U.S., only in military arsenals, and is purchased from the U.S. Army Armament Materiel Command.[6]
Uses
The majority of TNT used in the United States is used by the Military, for its own use.[2] Trinitrotoluene is an explosive material. The earliest known use of TNT as a military explosive was in 1902. German Soldiers filled artillery shells with TNT, forming armor-piercing rounds that exploded after impact and after they had penetrated the armor. TNT is still a widely utilized explosive in the military, and in many different companies around the world. [1] It is still considered one of the most frequently used high explosive used by the military. [3] It is used in bombs and grenades, as well as filling shells and airborne demolition bombs.[6] The Radford Army Ammunition Plant is the current leader in the U.S. for TNT production, producing the majority used by the military.[1]
TNT was originally utilized as a yellow dye in 1863,[7] and is also used as a reagent in chemical synthesis,[1] and is used in the making of dyes and photographic chemicals.[6] It is used in the manufacture of munitions, [3] as a pure explosive, or in a binary mixture. The most common binary mixtures for TNT are cyclotols, octols, amatols, and tritonals.[6] There is also a small percentage used for industrial functions.[2] There are some companies, primarily demolition companies, that also use TNT in the United States. It is used to clear away debris in building foundations,[1] and also in deep well and underwater blasting.[6]
TNT vs. Dynamite
Dynamite and TNT are often mistakenly used interchangeably for the same thing. Many believe that TNT is the chemical name and dynamite is simply the common name, however, these two explosives are completely different.
Dynamite is essentially a white powder[8] wrapped in paper.[9] It was patented by Alfred Nobel in 1867. He was looking to find a way to make nitroglycerin more stable by combining the nitroglycerin with diatomaceous earth and sodium carbonate. He had his share of mishaps, however, including a factory explosion that killed his brother Emil. [8] Although people assume that dynamite contains TNT, it does not. Dynamite is actually an absorbent, nitroglycerin-soaked mixture. Because of this, it is extremely sensitive to shock. [9] Dynamite is highly explosive and detonates rapidly. TNT is far more stable and much lighter. Dynamite is also 60% denser than TNT. Dynamite is extremely difficult to transport because of its sensitivity and is unable to be used in its true form, needing to be kept in a mixture. Despite this fact, dynamite begins to sweat over time; releasing nitroglycerine which collects at the bottom of the container the dynamite is being store in. Because of this, it is common that the container be flipped during transportation. If the nitroglycerine were able to collect at the bottom of the container, crystals would begin to form and would offer a great deal of danger.[10]
TNT, on the other hand, was originally used as a yellow dye, but was used later on as an explosive because of a few convenience factors, including stability and safety.[9] The explosive form was discovered by German scientist Joseph Wilbrand in 1863. It is a high explosive, like dynamite is, but not as powerful, as well as being more difficult to detonate, which could be viewed as a pro or a con. It is far more stable than Dynamite, and is even able to be melted down in order to be poured into shell casings. However, despite being stable, it is highly toxic.[8] If it comes into contact with skin, irritation and discoloration can occur, with your skin turning yellowish orange. Longer exposure to TNT can lead to issues such as anemia, liver damage and spleen enlargement.[10]
Video
The United States Navy simulated a nuclear explosion utilizing 500 tons (1,000,000 pounds) of TNT in Hawaii in 1965 in order to test the resilience of Navy ships.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Johnsams. Three Common High Explosives And Their Properties Hub-Pages. Web. Last Updated July 30, 2010.
- ↑ 2.0 2.1 2.2 2.3 Trinitrotoluene pubchem. Web. Accessed January 9, 2015. Unknown Author.
- ↑ 3.0 3.1 3.2 3.3 Technical Fact Sheet–2,4,6-Trinitrotoluene (TNT) Environmental-Protection-Agency. Web. Published January 2014. Unknown Author.
- ↑ 4.0 4.1 2,4,6-Trinitrotoluene OSHA. Web. Accessed January 12, 2015. Author Unknown.
- ↑ 5.0 5.1 2,4,6-Trinitrotoluene Chem-Spider. Web. Accessed January 10, 2015. Unknown Author.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Author Unknown. Production-Import-Use-and-Disposal ASTDR. Web. Accessed January 9, 2015. Cite error: Invalid
<ref>tag; name "astdr-pdf" defined multiple times with different content - ↑ Hiskey, Daven. TNT-was-originally-used-as-a-yellow-dye-not-to-make-bombs Today-I-Found-Out. Web. Accessed January 12, 2015.
- ↑ 8.0 8.1 8.2 tnt-vs-dynamite-whats-the-difference Mental-Floss. Web. Published August 29, 2008. Unknown Author.
- ↑ 9.0 9.1 9.2 Hiskey, Daven. TNT and Dynamite Are Not the Same Thing Today-I-Found-Out. Web. Accessed January 25, 2015.
- ↑ 10.0 10.1 Kumar, Manisha Difference Between TNT and Dynamite Difference-Between. Web. Published October 30, 2009.
| ||||||||||||||





