Stibine
| Stibine | ||
|---|---|---|
| ||
| General | ||
| Systematic name | Stibine | |
| Other names | Antimony trihydride | |
| Molecular formula | H3Sb | |
| SMILES | [Sb] | |
| Molar mass | Molar mass::124.784 g/mol | |
| Appearance | colourless gas | |
| CAS number | CAS number::7803-52-3 | |
| Properties | ||
| Density and phase | Density::5.48 g/L, | |
| Solubility in water | insoluble | |
| Melting point | Melting point::-88°C | |
| Boiling point | Boiling point::-17°C | |
| Structure | ||
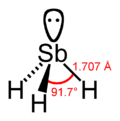
| Molecular shape | Trigonal pyramidal | |
| Hazards | ||
| MSDS | Material safety data sheet | |
| Main hazards |
Harmful (Xn) | |
| NFPA 704 | ||
| Flash point | Flammable gas | |
| R/S statement | R: R20/22 R50/53 S: (S2) S61 | |
| RTECS number | WJ0700000 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | ||
Stibine is a chemical compound that remains a gas with no color. The chemical formula for Stibine is H3Sb, and is also known as Antimony hydride. It wasn't until 1837 that Lewis Thomson and Pfaff ultimately discovered Stibine. It took quite a long time to find properties of this toxic gas because a suitable synthesis was not available at the time. A man named Francis Jones tested several synthesis methods in 1876, but Alfred Stock determined the properties in 1901.[1]
Properties
Stibine is a colorless gas that produces a very strange odor. This happens when antimony containing lead charges with a battery. Stibine's physical state is gas that occupies the same odor of hydrogen sulfide. But seemingly there was no odor threshold data located.[2] The properties of Stibine are very similar to those of Arsine. It is very unstable and typical of heavy hydride. The preparation of Stibine can be created by the reaction of Sb3+ sources with H- equivalents.[1]
Production
Stibine comes from Antimony and can be produced in different ways. It can be produced when strong reacting agents react with acid solutions of Antimony. It can also be produced when batteries containing lead are charged. These processes unfortunately require Nascent Hydrogen which makes it a bit more difficult to produce. Stibine can be created by dissolving Magnesium-Antimony or Zinc-Antimony in diluted hydrochloric acid. The formation of lead plates is another way in which Stibine can be produced. [2]
Uses
Stibine is a man-made compound which makes the abundance of it, at a minimum. Some of the uses of Stibine are prominently used in the semiconductor industry. Arsine and Stibine are both used in making microchips. It is also used in industrial processes such as welding, soldering, and galvanizing. Stibine is prepared for industrial use by reaction of Antimony compounds with water or hydrochloric acid. Stibine is also used extensively as doping agents. Which means it is added to a pure substance to produce a deliberate change especially with semiconductors and laser crystals. [3] It could also be used in glass dyes or light emitting diodes, but this is yet to be determined. [4]
Hazards and Effects
Stibine can cause death from pulmonary congestion because it is an eye and respiratory irritant. It is also a hemolytic poison which goes into the blood and infects it. Humans get exposed to Stibine in factories where it is produced but suffer more effects from it with Arsine and Sulfuric acid. More harmful effects can occur when breathing in Stibine at production sites, especially when it is produced from the charged batteries.[2] Stibine can be very harmful for fires, the environment, and damage to red blood cells. The release of Stibine exposure usually occurs through activities including welding and soldering. When Stibine is inhaled, it enters the blood by the lungs, while there it attacks and damages red blood cells. Some short term exposure to Stibine and Arsine might cause some of these symptoms: weakness, headache, drowsiness, sickness, abdominal pain, shortness of breath, yellow skin and eyes and red urine. Exposure to high concentrations of this chemical can cause conciseness and death. When people survive a short-term exposure it still can leave long term effects including kidney damage, confusion, memory loss, numbness and pain in the extremities. All of these effects depend on the amount and situation on which Stibine is consumed or exposed. Stibine exposure is very rare to because it is only in a couple production industries, and the public will not be exposed to a high enough level of it. [4]
References
- ↑ 1.0 1.1 Hangzhou weiku information & Technology Co.. Stibine LookChem. Web. Accessed 30 January 2012.
- ↑ 2.0 2.1 2.2 Author last, first. This is the preparation formula for Stibine, 4 SbCl3 + 3 NaBH4 → 4 SbH3 + 3 NaCl + 3 BCl3 Page-AEGLs EPA. Web. 6/2008 .
- ↑ Wiley, John. Doping Agent. Your Dictionary. Web. Accessed 29 January 2012.
- ↑ 4.0 4.1 Foxall, K. Arsine and Stibine HPA. Web. 2007.
| ||||||||||||||

