Hydrazine
| Hydrazine | |
|---|---|
| |
| General | |
| Systematic name | Hydrazine |
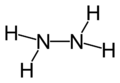
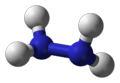
| Molecular formula | N2H4 |
| Molar mass | Molar mass::32.05 g/mol |
| Appearance | Colorless liquid |
| CAS number | CAS number::302-01-2 |
| Properties | |
| Density and phase | [[Density::1.01 g/cm3]], (liquid) |
| Solubility in water | infinitely miscible |
| Other Solvents | miscible with
polar organic solvents |
| Melting point | Melting point::1°C |
| Boiling point | Boiling point::114°C |
| Viscosity | .9 cP at 25°C |
| Structure | |
| Molecular shape | pyramidal at N |
| Dipole moment | 1.85 D D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Toxic, causes burns. |
| NFPA 704 | |
| Flash point | 37.78°C (closed cup) |
| R/S statement | R:45-10-23/24/25-34-43-50/53 S:53-45-60-61 |
| RTECS number | MU7175000 |
| Related compounds | |
| Related Hydrides | |
| Related compounds |
monomethylhydrazine |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Hydrazine is an inorganic compound that has many uses such as rocket fuel or as a powerful reducing agent. Hydrazine is also very toxic and unstable, without cautious and careful handling of this substance, bad things could occur. Contact with Hydrazine, may cause skin irritation in many places and even coma and seizures in humans.
Properties
Hydrazine is a compound similar to ammonia, having similar chemical and physical properties. Hydrazine is so similar to ammonia because it is actually made up of two ammonia molecules. Two ammonia molecules combine and lose one hydrogen atom, per molecule, to form a Hydrazine molecule.
Anhydrous Hydrazine is a highly polar and combustible substance. The combustion of Hydrazine decomposes into its original components such as ammonia and leftover hydrogen and nitrogen atoms. Porous substances that come in contact with Hydrazine may have a spontaneous reaction leading it to ignite in the air. Some of these reactions that include Hydrazine may have an explosive outcome.
More physical and chemical properties can be found in the column on the right of the page.
Occurrences
When it comes to Hydrazine, natural occurrences are rare. Hydrazine supposedly has occurred naturally in a few places, but these are just assumptions.
Natural Occurrences
In 1974, a tobacco company reported that Hydrazine was created through one of their nitrogen based processes. Later studies showed that Hydrazine may occur naturally in biological nitrogen fixation. Even though natural occurrences of hydrazine are scarce, hydrazine can be derived from many compounds, some of which are natural. These compounds include: gyromitrin, iproniazid, hydralazine, phenelzine, 1,1-dimethylhydrazine, 1,2-dimethylhydrazine, and phenylhydrazine. All of these can form or be transformed into a hydrazine molecule. For instance, gyromitrin comes from a widely produced mushroom species that can be metabolized into a form of hydrazine. Phenylhydrazine, in fact, was the first hydrazine that was discovered.
Synthetic Occurrences
In 1889, Theodor Curtius synthesized the first Hydrazine compound through the use of a continuous circuit. Hydrazine is produced in a process called the "Olin Raschig process", through the use of sodium hypochlorite and ammonia. This process was developed in 1907. This method relies on the fact that chloramine will react with ammonia.
Hydrazine can also be produced through a couple of the steps of synthesizing acetone.
Uses
Hydrazine is a extremely powerful reducing agent. Other uses include: rocket fuel, production of spandex fibers, blowing agent, solder, fluxes, and photographic developers. In the beginning of World War II, Hydrazine was starting to be used in rocket fuel. Hydrazine is also used in single propelled boosters on the side of the aircraft.
Hydrazine is also a keystone in finding and producing heterocyclic compounds.
References
- Hydrazine Wikipedia
- Hydrazine SafetyChemical Safety
- Hydrazine PropertiesProperties
| ||||||||||||||

