Gallium arsenide
| Gallium arsenide | |
|---|---|
| |
| General | |
| Systematic name | Gallium arsenide |
| Molecular formula | GaAs |
| Molar mass | Molar mass::144.645 g/mol |
| Appearance | Gray cubic crystals |
| CAS number | CAS number::1303-00-0 |
| Properties | |
| Solubility in water | < 0.1 g/100 ml (20 °C) |
| Melting point | 1238 °C (1511 K) |
| Structure | |
| Molecular shape | Linear |
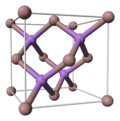
| Crystal structure | Zinc Blende |
| Hazards | |
| MSDS | Material safety data sheet |
| Main hazards | Toxic (T)
Dangerous for the environment (N) |
| NFPA 704 | |
| R/S statement | R: R23/25, R50/53 S: (S1/2), S20/21, S28, S45, S60, S61 |
Gallium arsenide is a compound two elements: gallium and arsenic. It has formula GaAs. Gallium arsenide is used in lasers, LEDs, microwave generators and other products that produce light. A chip is used in semiconductors made from gallium arsenide require less electric power and process data faster than chips made from silicon. [1] [2]
Properties
Gallium Arsenide has properties similar to silicon but it is better than silicon. It has a high electron mobility and saturated electron velocity. Therefore, it can function at frequencies in excess of 250 GHz. This is a lot of data and works faster compared to normal home computers that has CPU and only has a speed of two to three GHz. Therefore, GaAs devices make less noise than silicon devices. Also GaAs devices work in higher power level than those made from silicon because it is insensitive to heat and has higher breakdown voltages. [3]
Occurrences
Gallium arsenide is compound of two elements, gallium and arsenic. When these two individual elements combine, they forms Gallium arsenide. Gallium is rarer than gold. It does not occur in pure form in nature, but is found trace amounts from bauxite and zinc ores in form of Ga (III) salt. Gallium is a by-product of the smelting of other metals especially from aluminum and zinc. It is a soft silver metallic metal which is one of three metal melt to liquid at room temperature.[4] Unlike gallium, arsenic is a common element that can be easily found. It is a notoriously poisonous metalloid and has many different form. Sometimes it is yellow form of molecular non-metallic and seometimes it is several black and grey form of metalloid. Arsenic is commonly found in metals such as silver, cobalt, and nickel. Arsenopyrite is the most common arsenic-bearing mineral and is found in arsenides of metals such as cobalt, silver, and nickel.[5]
Uses
Gallium arsenide has been used in light-emitting diodes (LEDs), field-effect transistors (FETs), integrated circuits (ICs), laser, and other electronic devices that use light. The world uses electricity everyday but the expensive fossil fuel is limited and causes serious pollution to the world. To prevent this, people start to find way to use environmental energy that is effective and renewable energy. One of energies is solar energy and so far scientist find that Gallium Arsenide in solar cells is one of best compounds for improved electrical yield. Gallium Arsenide is used in multifunction and high-efficiency solar cells because it has some characteristic that make it suitable for them.
Advantage- First thing is that each solar cell of GaAs can handle 1.43 eV. Its absorptivity is very high, therefore it only needs few microns thick to absorb sunlight when crystalline silicon requires a thick layer of 100 microns or more. Also alloys made from GaAs using aluminum, antimony, phosphorus, indium have characteristics of great flexibility. Sometimes the cell temperature goes high but unlike silicon cells, GaAs cells are insensitive to heat. Lastly it is highly resistant to radiation damage. This is one reason it works well for space application. Unlike the silicon cell which is limited to variation in the level of doping, Gallium arsenide and its alloys as Pv cell materials provide designers with a way to make a very wide range of designs. Because of it designers are easier to control the generation and collection of electrons and holes.
Barrier- However like others, there is a greater barrier to Gallium Arsenide. A single-crystal GaAs substrate cost very high because Gallium is rarer than gold. Therefore, to reduce the cost, researchers are testing new things. They place GaAs cells on cheaper substrates, grow GaAs cells on a removable and reusable GaAs substrate, and make GaAs thin films.
Future
We cannot fully converts the sunlight to the actual electricity. So far, 30 percent of sunlight is top level that can be converted to the electricity. Scientists are still working to improve the conversion efficiency of the solar cell. However, there are a few types of solar cells that can get 40 percent of energy from the sunlight. The efficiency level can be changed depending on what material used. Recent past, the record shows people improve the efficiency level. On Dec. 5th, 2006, Boeing produced a high end solar cell that had 40.7 percent sunlight to electricity conversion rate. Also, on July 23, 2007, with crystalline silicon solar cell, the team from the University of Delaware broke the record by 42.8 percent from the sunlight. As a result, there are some things such as house and car that use most of their energy from solar energy. In the future, there will be a solar cell that has higher efficiency level and more products which is used solar energy.[9]
References
- Gallium Arsenide facts and figures, unkonwn
- Gallium Arsenide, unkonwn
- Solar Energy Technologies Program: Single-Crystalline Thin Film, unkonwn
- Galliu Arsenide, unkonwn
- Gallium arsenide solar cells - Appropedia: The sustainability wiki, Mutiple writer
- Gallium arsenide - Wikinfo, Mutiple writer
- Gallium, Mutiple writer
- Arsenic, Mutiple writer
| ||||||||||||||


