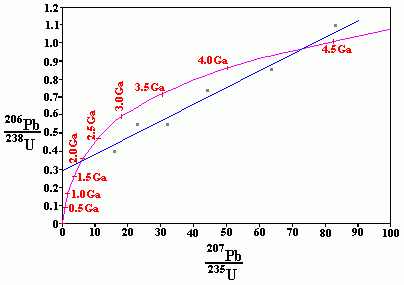
Concordia dating
Concordia dating is a form of uranium/lead dating that uses a concordia diagram like the one above. The assumption is that when zircons crystallize they lose all of their lead and as long as the crystal remains closed its lead/uranium ratios should follow curve in the chart above. It is known (experimentally) that synthetic zircon under certain conditions reject Pb almost completely, and under certain other conditions can incorporate up to 3% Pb during crystallization. (Refer Chemical Geology Volume 141, Issues 1-2, 29 August 1997, Pages 19-31). Different samples of zircon may have different concentrations of U and Pb initially.
It is further theorized that since all isotopes of the same element are chemically identical, they should be removed in proportional amounts, forming a straight line on the concordia diagram, that crosses the concordia curve at both the crystallization and the contamination date. Loss of uranium moves the point up and to the right, while a loss of lead moves the point down and to the left.
The straight line can be explained by natural mixing. Theoretically, if X1 and X2 are isotopes of an element X, and Y1 and Y2 are isotopes of element Y, the plot of X1/Y1 vs. X2/Y2 from different samples of the solid will be a straight line passing through the origin. Measurements from La Virgen volcano by A.K. Schmitt et al. (Journal of Volcanology and Geothermal Research, Vol 158, pg 288, 289) correlate well to a straight line through the origin.
Consider the experiment of mixing oil in water. Not all the oil droplets would be the same size. However if the carbon in oil contained 75% 12C and 25% 13C, then this carbon ratio would be preserved in every single oil droplet. Assume that the oxygen in oil and water has 60% 16O and 40% 17O.
Now assume we do an experiment where we solidify this oil-water mixture by freezing and collect 10 samples of the solid. Where there were big oil droplets, there would be a high ratio of 12C/16O and 13C/17O. Where there were small oil droplets, there would be a small ratio of 12C/16O and 13C/17O (less oil implies less 12C and 13C). If we plotted the 12C/16O ratio on the y axis, and the 13C/17O ratio on the x axis, we would obtain a straight line with the slope of (12C/13C)/(16O/170). A sample not containing any oil will fall on the origin since it does not contain carbon.
In reality you don't always get a nice neat line, showing that reality is more complicated than indicated by the theory. Furthermore, contamination can total reset the "clock", providing a way to explain data that does not fit the theory.
For Concordia dating the samples must have both the crystallization and the contamination dates, so this provides yet another way to explain data that does not fit the theory.
Concordia dating is based on the following assumptions.
- Radiogenic lead is from the in-situ decay of Uranium. Some zircon crystals have a 231Pa/235U activity ratio of 2. (Refer American Mineralogist, Volume 92, pages 691-694, 2007) This implies that for every 207Pb atom that is being produced from 235U, there is another 207Pb atom being produced that is not originating from in-situ decay of 235U. (Other than 231Pa, there are many other isotopes in the decay chain of 238U and 235U. If you know of some study that has actually checked whether the other isotopes of the decay chain of Uranium in zircon are in equilibrium, please link that study to this article.)
- All lead is removed from zircons when they crystallize, such that there are no daughter isotopes present in the original sample. If zircons cooled and crystallized faster than they are thought to have done, then they could have had original lead, and that would throw off the entire process.
- All isotopes of the same element are removed in proportional amounts. However, since lighter isotopes move faster than heavier ones at a given temperature, there would be a tendency for lighter isotopes to be removed a little faster.
- That the decay rates are constant. However, there is evidence of accelerated decay in the past.
Above is a concordia diagram based on accelerated decay and Biblical creation. While it is not a precise diagram, it illustrates the basic effect that accelerated decay and Biblical creation would have on concordia dating.
Given concordia dating's questionable assumptions and the ability of the theory to explain dates that don't fit the theory, it is reasonable to conclude that concordia dating is not reliable, as it is claimed to be.
Related References
- The age of Australian uranium
- Geol. 655 Isotope Geochemistry
- Mythology of Modern Dating Methods book by John Woodmorappe. 1999
 Browse |