Boric acid
| Boric acid | |
|---|---|
| |
| General | |
| Systematic name |
Boric acid Trihydroxidoboron |
| Other names |
Orthoboric acid |
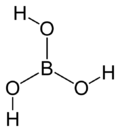
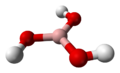
| Molecular formula | B(OH)3 |
| SMILES | OB(O)O |
| Molar mass | Molar mass::61.833 g/mol |
| Appearance | White crystalline solid |
| CAS number | CAS number::10043-35-3 |
| Properties | |
| Density and phase | Density::1.435 g/ml, solid |
| Solubility in water | 5.7 g/100 ml (25°C) |
| Melting point | Melting point::169°C(decomp.) |
| Boiling point | Boiling point::300°C(decomp.) |
| Acidity (pKa) | 9.24 |
| Structure | |
| Molecular shape | Trigonal planar |
| Crystal structure | triclinic |
| Dipole moment | 0 D |
| Hazards | |
| MSDS | Material Safety Data Sheet |
| Main hazards | Irritant in case of skin or eye contact |
| NFPA 704 | |
| Flash point | nonflammable |
| R/S statement | R: 36, 38, 40, 62 S: 24, 25 |
| RTECS number | ED4550000 |
| Related compounds | |
| Related compounds | Boron trioxide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Boric acid is a weak acid that was first prepared by Wilhelm Homberg (1652-1715) by combining borax and the action of mineral acids. At the time, the compound was given the name sal sedativum Hombergi, meaning "sedative salt of Homberg.[1] The ancient Greeks began using it to make their fabrics flame-retardant. Since then, this compound has proven itself to be extremely useful. Often found in a white powdery form, boric acid is used in a vast array of applications in the commercial, agricultural, and even nuclear fields. This eclectic acid is even known for its deadly effectiveness as a natural insecticide.[2]
Properties
The term boric acid refers to three compounds: metaboric acid (HBO2 or B2O3·H2O), tetraboric acid (H4B4O7 or B2O3·H2O), and orthoboric acid (H3BO3 or B2O3·3H2O). Both metaboric and tetraboric acid form when orthoboric acid dehydrates above 170°C and 300°C. When metaboric acid and tetraboric acid dissolve, they revert back to the original orthoboric acid.[3] Thus, orthoboric acid is the main compound referred to by the term boric acid.[4] This compound is generally found as a crystalline powder. The crystal structure of the acid is triclinic, meaning its crystal system has 7 lattice points.[5] The layers of molecules are held together by hydrogen bonds. Two adjacent layers of molecules are connected by 318 pm. of hydrogen bonds.[6] Boric acid is usually white, but its color ranges from clear to opaque.[7] It is poorly soluble in room temperature water, but easily dissolves in hot water, alcohol, and glycerine.[8] This hygroscopic compound is odorless and usually stable, but is slightly volatile in steam.[9] Boric acid is not a strong acid, but is considered acidic due to its interaction with water molecules.[10][11]
Occurrences
Boric acid is found in a variety of places. Because it is derived from boron, a naturally- occurring element, it can be found in nature as well as in the scientific laboratory.[12] The major source of boric acid is in volcanic areas, such as at steam vents or volcanoes. In Tuscany, the Lipari Islands, and Nevada, the free acid has been found mixed with steam from fissures in the ground.[13][14] Boric acid is a common constituent of several minerals such as borax, boracite, boronatrocaicite, and colemanite. Many plants and almost all fruits have been reported to contain boric acid. Its presence and the presence of its salts have even been detected in seawater, where it contributes to the absorption of low frequency sound. Borax is also easily created in a laboratory by treating borax with sulfuric acid or by the action of mineral acids.[15][16]
Uses
Boric acid is a versatile compound that has served a variety of unique purposes throughout history. In ancient times, the Greeks used it to fireproof cloth. In the late 1800's and early 1900's, it was used as a food preservative until its harmful effects were discovered. It even was used as an ingredient for diaper rash creams, but babies became sick because of it.[17] Despite its mildly hazardous qualities, it is still used today. Mainly, it is used to make borate salts, such as borax.[18] However, it serves a large variety of other purposes as well.
Medicinal
When boric acid is in a solution, it is only slightly acidic. Therefore, it can be used for both cosmetic and medicinal purposes. In a solution, it is utilized as an astringent antiseptic for minor burns or cuts.[19] It's even mild enough to be used as an acne treatment or emulsifier. [20][21][22] Boric acid in solution is only slightly acidic and acts as a nonirritating, slightly astringent antiseptic, mild enough to be used as an eyewash. It also treats yeast and fungal infections, including athlete's foot.[23]
Preservation
Boric acid is hygroscopic, meaning it takes moisture from the air. This property allows it to prevent and destroy fungal growth in citrus fruits and rot in wood. In combination with an ethylene glycol carrier, boric acid can protect the wood against fungal and insect attack.[24]
Nuclear
Boric acid is also used in nuclear power plants. It acts to slow down the rate of nuclear fission. The rate of fission depends on how many neutrons are present. Boric acid, when circulated throughout the nuclear reactor, reduces the probability of a neutron's survival, thus hindering the rate of fission. [25]
Commercial Use
Boric acid plays a role in many commercial industries. The jewelry industry uses boric acid to create artificial gems and to protect the beauty of jewelry during smoldering. When combined with alcohol, it can reduce surface oxidation and firescale from forming on metals during certain operations.[26] It is also used in the production of steel, fiberglass, and glass, such as LCD flat panel displays.[27][28] The boron fibers add a tensile strength to the material to make it strong. Ceramic companies use boric acid to reduce the melting point of the product and prevent cracking or distortion. In this way, it is an essential ingredient for both the ceramic product and its glaze.[29] Another important commercial use of boric acid is its ability to make cloth and other products flame-retardant.[30]
Other
Aside from the main uses of boric acid, the compound also serves in unexpected areas such as pyrotechnics , toys, and even Indian games. For example, Silly Putty, a popular product for children, was originally made by mixing silicone oil and boric acid. Boric acid is also added to fireworks to prevent an amide-forming reaction between aluminium and nitrates. Indians dust Carrom boards with boric acid to reduce friction. When added to salt, boric acid can help in the curing of cattle hides and other skins. [31][32]
Insecticide
The most well-known application of boric acid is its household use as an insecticide. The compound was first registered as an insecticide in 1948 in the U.S. to control many insects, including termites, cockroaches, and ants.[33] The compound is considered to be a safe product, even for household kitchens. Paste or gel forms of boric acid can be found in most hardware stores. This makes it convenient for the user to dab under problem areas. When mixed with sugar, boric acid serves as an excellent ant bait. The ant carries back the poison to its nest, killing all the other ants that eat it.[34] Thin lines of boric acid are sprinkled on problem areas, mainly cracks, crevices, and corners of rooms like kitchens. This product should not be placed on any surfaces used for food preparation, as boric acid is still somewhat toxic to humans.[35][36]

Boric acid acts as a stomach poison that affects the metabolism of an insect. The dry powder is also abrasive to the insect exoskeleton. The boric acid kills insects by dehydrating them. The insect is first attracted to the compound, sometimes by sugar or other attractants mixed with the boric acid.[37] The insect will eat some of the compound and/or carry some back on their body to the nest or colony. Often, the insect will eat the powder from its own body when grooming.[38] Others in the nest or colony will also eat it. The poison works as soon as it is ingested. Because it is a dessicant, the powder will remove the moisure from the body of the pest, slowly dehydrating them. This can lead to severe dehydration, electrolyte metabolism, metabolic acidosis, and death to the insect. Because it works so slowly, this also means that the colonies will die at their nest and not at the site where the boric acid was placed. One application can effectively eliminate the pest for up to a year. Another major benefit to using boric acid is that the insects have no way of building an immunity to it, because it works by dehydrating the insects. [39][40]
References
- Safety data for boric acid The Physical and Theoretical Chemistry Laboratory at Oxford University.
- Boric acid Wikipedia.org.
- Boric Acid Chemicalland21.com.
- Opinion & Information on Boric Acid By Michael R. Cartwright, Sr. Dvfmastiff.net.
- Borichtech. National Pesticide Information Center.
- Boric acid history People For Clean Beds.org.
- Boric Acid Microsoft® Encarta® Online Encyclopedia 2008.
- Boron & compounds fact sheet Australian Government. Department of the Environment, Water, Heritage, and the Arts.
- Cockroach Elimination University of Kentucky: Entomology.
- Boric Acid Powder, Borate, Insect Control, and Wood Preservative Livingwithbugs.com.
| ||||||||||||||





