Tabun
| Tabun | |
|---|---|
| |
| General | |
| Systematic name | Organophosphorous compound |
| Other names | Ethyl dimethylplosphoramidocyanidate
EA1205 |
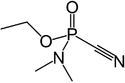
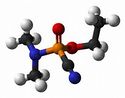
| Molecular formula | C5H11N202P |
| Molar mass | 162.13 g/mol |
| Appearance | Colorless to brown liquid |
| CAS number | 77-81-6 |
| Properties | |
| Density and phase | Density: 1.073 g/ml, 25 °C |
| Solubility in water | 9.8 g/100 ml (25°C) |
| Melting point | -50°C |
| Boiling point | 247°C |
| Acidity (pKa) | Unknown |
| Basicity (pKb) | Unknown |
| Viscosity | 1 cP at 20°C |
| Hazards | |
| MSDS | Material safety data sheet |
| Main hazards | Highly toxic, poisonous, and flammable. |
| NFPA 704 | |
| Flash point | 78°C(172 °F; 351 K) |
| R/S statement | R: Unknown S: Unknown |
| RTECS number | TB4550000 |
| Related compounds | |
| Related compounds | Sarin, Soman, Cyclosoman |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Tabun is an extremely toxic chemical substance that was originally made to controlinsects. By the time of WWII Germany started mass producing Tabun to use a chemical weapon. Tabun is very toxic and it is flammable. At the end of World War II, Tabun was rarely ever seen in war but it still was used although but not as much as it was used in WWII. Tabun hasn't been used to much because of new chemical weapons which seemed to be much more effective than Tabun but it was used only once after WWII. The second time Tabun was used in war was in the Iraq war. Iraq had deployed different kinds of chemical weapons against Iranian ground forces. But it wasn't as commonly used as mustard gas and a couple different chemical weapons but it was still used. Producing or stockpiling tabun was banned by the 1993 Chemical Weapons Convention.
Properties
Chemical
The Chemical properties for Tabun are decomposition, evaporation, and combustion.[1]
Physical
Tabun is a colorless to brown liquid with a faint fruity odor and was used as a chemical warfare agent. The boiling point of Tabun is 247.5 °C (477.5 °F; 520.6 K), the melting point is −50 °C (−58 °F; 223 K), and the flash point is 78 °C (172 °F; 351 K). The solubility is readily soluble in organic solvents, in water the soluble is 9.8X10+4 mg/L at 25 deg C Abstract. The density of Tabun is 1.073 g/mL at 25 deg C, and the vapor density is 5.63. The vapor pressure is 0.07 mm Hg at 77° F(0.07 mm Hg at 25 deg C).[2]
Synthesis
Tabun was made on an industrial scale by Germany during World War II, and it was based on the process developed by Dr. Gerhard Schrader. The chemical agent was produced in a factory Germany code named "Hochwerk", had manufactured at least 12,000 metric tons of this agent between 1942 and 1945. The manufacturing process consisted of two steps, the first being reaction of gaseous dimethylamine with an excess of phosphoryl chloride, yielding dimethylamidophosphoric dichloride (also known as "D 4"), and dimethylammonium chloride. The "D 4" thus obtained was purified by vacuum distillation and thereafter transferred to the main Tabun production line. Here it was reacted with an excess of sodium cyanide, it was then dispersed in dry chlorobenzene, yielding the intermediate dimethylamidophosphoric dicyanide and sodium chloride then, absolute ethanol was added, reacting with the [dimethylamidophosphoric dicyanide] to yield Tabun and [hydrogen cyanide]. After the reaction, the mixture (consisted with about 75% chlorobenzene and 25% Tabun, along with insoluble salts and the rest of the hydrogen cyanide) was then filtered to remove the insoluble salts and vacuum-distilled to remove hydrogen cyanide and excess [chlorobenzene]. This is how Tabun was made in World War II but today it is illegal to make Tabun worldwide.[3]
Uses
The original purpose of Tabun and other related compounds was to control insects. These pesticides were similar to organophosphates in their action on the nervous system. But Tabun and the other human-made nerve agents was much more potent than the organophosphates, and so quickly, it was categorized as chemical weapons. It was used in World War II by using it to contaminate food, poison water, or it could absorbed through skin (only in liquid form).[4]
Hazards
Since Tabun was used as a chemical warfare weapon it is very toxic and poisonous to humans. The health hazard is that this material is toxic by inhalation and by absorption through skin and eyes. The lethal dose for humans may be as low as 0.01 mg/kg. Tabun is a nerve agent; it acts as a cholinesterase inhibitor. Flammable 2nd degree, Reactive 1st degree, the potential of it causing a fire is combustible. The fire hazards sparks, flames, and sources of ignition. Keep out of water sources and sewers. Hydrolysis forms hydrogen cyanide when heated to decomposition, it emits very toxic fumes of oxides of phosphorus and nitrogen. Avoid water and acids can react with oxidizing material.[1]
References
- ↑ 1.0 1.1 Tabun "Pubchem". Web. Accessed on May 3, 2017. Unknown Author.
- ↑ Tabun "Pubchem". Web. Accessed on May 2, 2017. Unknown Author.
- ↑ Tabun (nerve Agent) - Synthesis "Liquisearch". Web. Accessed on April 23, 2017. Unknown Author.
- ↑ Tabun "Encyclopedia.com". Web. Accessed on April 23, 2017. Unknown Author.
| ||||||||||||||



