Sodium sulfate
| Sodium sulfate | ||
|---|---|---|
| ||
| General | ||
| Systematic name | Sodium sulfate | |
| Other names | Thenardite (mineral) Mirabilte (decahydrate) Sal mirabilis (decahydrate) Glasuber's salt (decahydrate) | |
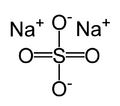
| Molecular formula | Na2SO4 | |
| SMILES | [Na+].[Na+].[O-]S([O-])(=O)=O | |
| Molar mass | [[Molar mass::142.04 g/mol (anhydrous) 322.20 g/mol (decahydrate)]] | |
| Appearance | white crystalline solid, hygroscopic | |
| CAS number | CAS number::7727-73-3 | |
| Properties | ||
| Density | [[Density::2.664 g/cm3 (anhydrous) 1.464 g/cm3 (decahydrate)]] | |
| Solubility in water | 47.6 g/L (0°C) ethanol | |
| Melting point | [[Melting point::884°C (anhydrous) 32.4°C (decahydrate)]] | |
| Boiling point | Boiling point::1429°C (anhydrous) | |
| Structure | ||
| Crystal structure | orthorhombic or hexagonal (anhydrous) monoclinic (decahydrate) | |
| Hazards | ||
| MSDS | Material safety data sheet | |
| Main hazards | Irritant | |
| NFPA 704 | ||
| Flash point | Non-flammable | |
| Related compounds | ||
| Other anions | Sodium selenate Sodium tellurate | |
| Other cations | Lithium sulfate Pottassium sulfate Rubidium sulfate Caesium sulfate | |
| Related compounds | Sodium bisulfate Sodium sulfite Sodium persulfate |
|
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | ||
Sodium sulfate is a sodium salt which has the formula Na2SO4. It is known as Glauber's salt, named after the German chemist Johann Rudolf Glauber(1604-1670) who discovered it in 1625. When he discovered it, he called it sal mirabilis. It appears as an anhydrous, decahydrate, and heptahydrate forms. Sodium sulfate is a white crystalline solid when it appears as anhydrous. When it appears as decahydrate, it has formula of Na2SO4·10H2O. When it is cooled, it appears as heptahydrate. It is usually stays stable. Sodium sulfate can be produced naturally or chemically. When it is naturally produced, it is usually found in Great Salt Lake. When it is chemically produced, it is usually reacted with other chemicals. Sodium sulfate is usually used for making detergents, textiles, heat storage, and others. [1]
Properties
Sodium sulfate has a density of 2.664 g/cm3 (anhydrous) and 1.464 g/cm3 (decahydrate). Its melting points are 884°C (anhydrous) and 32.4°C (decahydrate). Boiling point of sodium sulfate is 1429°C (anhydrous). It is non-flammable and appears as white crystalline solid. Sodium sulfate usually stays stable. Even if it is heated, it does not decompose. At normal temperatures, Sodium sulfate does not react with reducing and oxidizing agents. Sodium sulfate can become sodium sulfide when temperatures are raised. When it is dissolved in water, sodium sulfate is a neutral salt with a pH of 7. The reason is that sodium sulfate originates from a strong base (sodium hydroxide) and a strong acid (sulfuric acid).
Sodium sulfate can react with sulfuric acid in aqueous solution:
Na2SO4(aq) + H2SO4(aq) --> 2 NaHSO4(aq)
Na2SO4 is considered as a typical ionic sulfate since it has Na+ ions and SO42- ions.
Precipitates can be produced by aqueous solutions with combination of salts of barium or lead:
Na2SO4(aq) + BaCl2(aq) --> 2 NaCl(aq) + BaSO4(s)
Unlike other compounds, sodium sulfate has special solubility characteristics in water. If one adds chloride into sodium sulfate, the solubility will be lower. People can explain unusual characteristics of sodium sulfate with hydration. The reasons is that 32.4 °C correlates with the temperature where Glauber's salt transforms into an anhydrous solid phase and a sulfate liquid phase.[2] When temperature is increased, the solubility of sodium sulfate will increase sharply too. However, solubility will start to decrease slowly after it reaches certain temperature which is around 33°C. The decagydrate crystals gives the anhydrous salt by decomposing at around 33°C. Moisture in the air is absorbed by the anhydrous salt promptly to give the efflorescence decahydrate crystals.[3]
Because of similar properties, sodium sulfate is often replaced by other compounds. Sodium sulfate can be replaced by sodium hydroxide (caustic soda) and emulsified sulfur for paper production. It also can be replaced by calcium sulfate and soda ash for glass production.[4]
Occurrences
Sodium sulfate can be produced naturally and chemically. About 400 million tons of sodium sulfate (12%) is found in the United States of America. China produces the most sodium sulfate. It is expected that one production company will produce over 4.8 million metric tons by 2013. Even though most of Sodium sulfate is produced in China, it is also produced in many other countries. Some amounts of sodium sulfate are created as by- product. Sodium sulfate that, produced chemically and naturally, are able to exchange with another. [4]
Naturally produced
In the United States, two companies usually extract natural sodium sulfate from plants in Texas and California. People can extract sodium sulfate from the brine waters of Searles Lake in California. The amount is estimated about 450 million metric tons. 12% of sodium sulfate are in the Great Salt Lake of Utah. This amount can translate sodium sulfate into 400 million tons. Washington, Nevada, and Wyoming are also states that have natural sodium sulfate. Not only the United States, but also other nations have natural sodium sulfate. These countries are: Mexico, Canada, Spain, Turkey, China, Italy, Egypt, South Africa, and Romania. This is why the United States imports sodium sulfate from other countries. [5]
Chemically produced
Whereas half of the world's sodium sulfate is found in the natural environment, the other half is usually chemically produced. There are several processes that can produce sodium sulfate. It is usually produced a by-product of other processes.[2]One of the most important chemical productions of sodium sulfate is hydrochloric acid production. In the Mannheim process, sodium sulfate is created either from sulfuric acid or sodium chloride. Also, it can be produced by Hargreaves process with sulfur dioxide. When produced as a result of these processes, sodium sulfate is called as salt cake.
- Mannheim process:
2 NaCl + H2SO4 → 2 HCl + Na2SO4
- Hargreaves process:
4 NaCl + 2 SO2 + O2 + 2 H2O → 4 HCl + 2 Na2SO4
The process by which surplus sulfuric acid is neutralized by sodium hydroxide, is the next major process of sodium sulfate. This process is usually applied and convenient labratory preparation.
2 NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2 H2O(l)
When sulfuric acid is added to sodium chromate solution, sodium dichromate is produced. Sodium sulfate is also formed by sodium dichromate. Then again, sodium sulfate can again be produced by the production of chelating agents, lithium carbonate, ascorbic acid, resorcinol, phenol, nitric acid, and, silica pigments. [1]
History
Sodium sulfate is also called Glauber's Salt. An apothecary and German chemist named Johann Rudolf Glauber(1604-1670) discovered Sodium sulfate in 1625 in Austrian spring water. When he first discovered it, he called it sal mirabilis which means miraculous salt. He named it because it has medicinal properties. The crystals in this salt were used for laxative purposes. In the 18th century, people started to use sodium sulfate for industrial production of sodium carbonate. [6]
Uses
Around 1995, people in United States of America used to sell sodium sulfate for around $70 per tonne. It was even sold for $90 for good qualities. This means that sodium sulfate was very cheap material. Today, sodium sulfate has become widely used materials. It is used for making detergents, soaps, glasses, heat storages, and others.
Detergent and soap
Since sodium sulfate is so cheap, people started using it to make detergent and soap. Probably people use sodium sulfate mostly for filler in powdered home laundry detergents. In Europe, total consumption of Na2SO4 was about 1.6 million tons. 80% was used as detergents.[2] The reason why people used sodium sulfate for home laundry detergents is whiteness of sodium sulfate. However, around late 1980, people started to use liquid detergents and superconcentrates. This fact is very important because liquid detergents does not require sodium sulfate. [6]
Glass
Sodium sulfate is not only used for detergent and soap, but also used for the glass industry. The glass industry in US uses about 30,000 tons. Sodium sulfate is used to get rid of small air bubbles from molten glass.This is called "a fining agent". It also prevents scum formation and fluxes the glass. The glass industry also says that glass is created by meting mainly sodium sulfate, a mixture of sand, and sodium carbonate.[6]
Heat Storage
People used Glauber's salt, the decahydrate, as a laxative. People also started to used it for heat storage in passive solar heating systems. This is really beneficial for people because it gives profits of the high heat of crystallization (78.2 kJ/mol). Also it gives benefir of unusual solubility properties.
Others
In Japan, Sodium is importantly used for textiles. It helps to decrease negative charges on fivers and this makes dyes penetrate evenly. Sodium sulfate is not like sodium chloride that it does not damage the stainless steel vessels. Other uses of sodium sulfate can be frosting windows, carpet fresheners, an additive to cattle feed, and starch manufacture. Lastly, anhydrous sodium sulfate, in the laboratory, is used for inert drying agent for oraganic. [2]
Different names for Sodium sulfate
- Mirabilite (decahydrate)
- Disodium monosulfate
- Sulfuric acid, Sodium salt
- Disodium sulfate
- Sulfuric acid, Disodium salt
- Natriumsulfat
- Thenardite (mineral)
- Glauber's salt (decahydrate)
- Sal mirabilis (decahydrate)
- Trona
- Salt cake
- Bisodium sulfate [4]
Safety
Since sodium sulfate is a compound, people need to know safety facts about sodium sulfate. People have to put sodium sulfate in a closed container. They also have to separate it from incompatible substances. Examples are strong acids, magnesium and aluminum. Sodium sulfate is not expected to be a health hazard when people touch with their skins. It can have a mild toxicity because of ingestion. It can cause purging, falling of blood pressure, fluid loss when it goes into water. The reason is its osmosis activity. People should not put this into their eyes. If people touch it with their skins, they should wash it off with water and soap. If it goes into their eyes, they should wash it off with running water. People should take medications if they see irritations has developed. Lastly, if people accidentally eat it, they should drink many glasses of water. [3]
Videos
References
- ↑ 1.0 1.1 Sodium sulfate Wikipedia.
- ↑ 2.0 2.1 2.2 2.3 Sodium sulfate Hans Lohninger, Wikipedia.
- ↑ 3.0 3.1 Sodium sulfate decahydrate etacude
- ↑ 4.0 4.1 4.2 Sodium Sulfate Minerals Zone
- ↑ Sodium sulfate Mineral Information Institute, Eoearth,January 20, 2008
- ↑ 6.0 6.1 6.2 Sodium sulfate Tititudorancea Cite error: Invalid
<ref>tag; name "example" defined multiple times with different content Cite error: Invalid<ref>tag; name "example" defined multiple times with different content - ↑ [1] Cristallerie, Youtube, March 18,2008
| ||||||||||||||





