Potassium sulfate
| Potassium sulfate | |
|---|---|
| |
| General | |
| Systematic name | Dipotassium sulfate |
| Other names | Potassium sulphate |
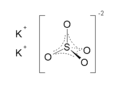
| Molecular formula | K2SO4 |
| SMILES | [K+].[K+].[O-]S([O-])(=O)=O |
| Molar mass | Molar mass::174.259g/mol |
| Appearance | White solid |
| CAS number | CAS number::7778-80-5 |
| Properties | |
| Density and phase | [[Density::2.66 g/cm3]] |
| Solubility in water | 111g/1L (20°C) 120 g/L (25°C) 240 g/L (100°C) |
| Melting point | Melting point::1,069°C |
| Boiling point | Boiling point::1,689°C |
| Viscosity | Not available. |
| Structure | |
| Crystal structure | orthorhombic |
| Hazards | |
| MSDS | Material safety data sheet |
| Main hazards | Irritant |
| NFPA 704 | |
| Flash point | Non-flammable |
| R/S statement | R: R22 S: S36 |
| RTECS number | TT5900000 |
| Related compounds | |
| Other anions |
Potassium selenate |
| Other cations | Lithium sulfate Sodium sulfate Rubidium sulfate Caesium sulfate |
| Related compounds |
Potassium hydrogen sulfate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Potassium sulfate is an inorganic crystalline salt with a chemical formula K2SO4 Another name for it is sulphate of potash, or the mineral form arcanite. Potassium sulfate’s is a white crystal or powder and odorless, bitter tastes, and nontoxic.
Properties
Potassium sulfate is mainly composed of potassium and sulfur. [1] Potassium sulfate has appearances of white crystal or powder. [2] Also, it has odorless, tastes bitter, and is nontoxic. However, If the temperature is going up to over 700 degrees, the Potassium sulfate separates with SO4 and K2. That point makes toxic gases. [1] Potassium sulfate is soluble in water because it is ionic compound. However, it is not soluble in ethanol. [2] When the water temperature is going up to 20 degrees, it has a solubility of about 111g/1L. However, if the water is boiling, the Potassium sulfate dissolves 240g/1L. Also, the glycerol is 1g/75ml. If Potassium sulfates is being used to make an aqueous solution, it becomes neutral. In addition, when the normal pressure or temperature, the Potassium sulfate can be stabled. [1] Also, if you dissolve or melt potassium sulfate in water, it conducts electricity very well.[2]
Occurrences
Potassium sulfate is found in the mineral of arcanite but it is not found easily. [3] The arcanite appears as a colorless and moist crystal. It occurs in the pure state of salt naturally. However, potassium sulfate can be found widely in the mineral of double salts with a combination of calcium sulfate, magnesium sulfate, and sodium sulfate. [4] These are minerals of potassium sulfate, Kainite [MgSO•KCl•HO], Schönite [KSO•MgSO•6HO], Leonite [KSO•MgSO•4HO], Langbeinite [KMg(SO)], Glaserite [KNa(SO)], Polyhalite [KSO•MgSO•2CaSO•2HO] Also, the minerals of potassium sulfate are abundant in the Stassfurt salt. When using the mineral of kainite, the potassium sulfate can be separated because kainite’s salt is less soluble in the water than other minerals. The other mineral of kierserite [MgSO•HO], if a person uses that mineral with the solution of potassium chloride, it can be produced as potassium sulfate as well. [3]
Uses
Potassium sulfate is used in various forms in industries ike fertilizer production, making potassium alum or potassium carbonate, and making glass. [5] Commonly, the Potassium sulfate is used in fertilizer because potassium is very important for plants and crop's nutriment. [6]In addition, Potassium sulfate is non chloride or less chloride than Potassium chloride [7] and it has 18% of sulfur. Those points make good fertilizer for crops. [8] Therefore, potassium sulfate is used in fertilizers instead of potassium chloride because some crops are very sensitive in chloride for example, berries and vines. [7] However, in the potassium fertilizers, Potassium chloride is most commonly used in agriculture (90%) [6] because it has the lowest price.[8] After that, Potassium sulfate, Potassium-magnesium salts and Potassium nitrate are commonly used in potassium fertilizers.[6]
History
Potassium sulfate had been already known by Glauber, Boyle and Tachenius in 14th century. Then after time passed, in the 17th century, the potassium sulfate produced with combination of acid salt and alkaline salt. After that, potassium sulfate called sal duplicatum or arcanuni in that time. Also, the potassium sulfate called different name as vitriolic tartar and Glaser's salt or sal polychrestum Glaseri because of the chemist Christopher Glaser. He was interested in medicine manufacture. Then he found how potassium sulfates can be used with medically. Therefore, in that time it also was called Glaser. [3]
Video
This video shows the between using Potassium sulfate(SOP) and Potassium chloride(MOP) for crop(Potato).
References
- ↑ 1.0 1.1 1.2 Potassium sulfate LookChem. Web. Accessed April 21, 2015. Author unknown.
- ↑ 2.0 2.1 2.2 Potassium sulfate weebly . Web. Accessed April 21, 2015. Author unknown.
- ↑ 3.0 3.1 3.2 Potassium sulfate Revolvy. Web. Accessed May 6, 2015. Author unknown.
- ↑ Potassium sulphate Industrial Project Report. Web. Accessed May 6, 2015. Author unknown.
- ↑ POTASSIUM SULFATE, ANHYDROUS MP BIOMEDICALS. Web. Accessed April 21, 2015. Author unknown.
- ↑ 6.0 6.1 6.2 Potassium fertilizers – Manufacturing process of Potassium fertilizers Guichon Valves. Web. Accessed April 21, 2015. Author unknown.
- ↑ 7.0 7.1 Sulphate of Potash (SOP) or Potassium Sulphate impact fertilizers. Web. Accessed April 21, 2015. Author unknown.
- ↑ 8.0 8.1 Potassium Fertilizers Plant & Soil Sciences eLibrary. Web. Accessed April 21, 2015. Author unknown.
| ||||||||||||||



