Potassium bicarbonate
| Potassium bicarbonate | |
|---|---|
| |
| General | |
| Systematic name | Potassium hydrogen carbonate |
| Other names | Potassium acid carbonate |
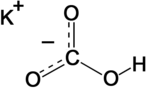
| Molecular formula | CHKO3 |
| SMILES | C(=O)(O)[O-].[K+] |
| Molar mass | Molar mass::100.115 g/mol |
| Appearance | White crystal |
| CAS number | CAS number::298-14-6 |
| Properties | |
| Density and phase | [[Density::2.17g/cm3]], solid |
| Solubility in water | 3.7g/100 ml (20°C) |
| Other solvents | insolubility in alcohol |
| Melting point | Melting point::100°C decomposition |
| Boiling point | Boiling point::Not applicable |
| Acidity (pKa) | 10.329, 6.351 (carbonic acid) |
| Hazards | |
| MSDS | Material safety data sheet |
| NFPA 704 | |
| Flash point | Not flammable |
| RTECS number | FG1840000 |
| Related compounds | |
| Other anions | Potassium carbonate |
| Other cations | Sodium bicarbonate
Ammonium bicarbonate |
| Related compounds | Potassium bisulfate
Potassium hydrogen phosphate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Potassium bicarbonate has a variety of uses. It is mostly used for Potassium supplements and Fire extinguishers. This compound is good in fire extinguishers because it is Fire retardant. Potassium bicarbonate has similar properties to Sodium bicarbonate and these two compounds are common to be found in fire extinguishers. Potassium bicarbonate is also insoluble in alcohol.
Properties
Potassium bicarbonate is insoluble in alcohol. Potassium bicarbonate is white crystal And is also non flammable. It is good for treating high blood pressure and low serum potassium levels. It is also known to reduce risks of stroke, osteoporosis, and kidney stones. Potassium bicarbonate has many similar qualities as Sodium bicarbonate, which is more commonly known as being baking soda.[1]
Synthesis / Occurrences
Potassium bicarbonate is created in two different ways. One way is adding potassium to bicarbonate. Another way to create it is by carbonating potassium hydroxide (KOH) into K2 CO3. Carbonation is done by injecting carbon dioxide (CO2) into an aqueous solution(soluble in water)of potassium hydroxide. [2]
Potassium bicarbonate is also found in the nature as a mineral called kalicinite. The mineral has the exact composition as potassium bicarbonate which is KHCO3. Kalincinite is found naturally in Canada, Russia, and the northeast part of North America. The form that is created in a lab is used more frequently than the mineral. This is because it is more cost effective to manufacture Potassium bicarbonate then to mine kalicinite.[1]
Uses
Potassium bicarbonate has a variety of different uses. In foods it is use for many different purposes. For example Potassium bicarbonate is in club soda to reduce the effervescence (foam or fizz) and in water to change the taste.[3] Also it is in many foods to help regulate the pH, a leavening agent, and used to preserve food color. It is used in animal feed to improve performance in meat and dairy animals and as a rumen buffer. Potassium bicarbonate put into the soil corrects the pH and reduces acidity. For potatoes it brings about a larger protein and starch crop. When used to fertilize tobacco, it improves color, burn properties, and reduces leaf size. Potassium bicarbonate is used in fire extinguishers. It is in dry powder extinguishers and automatic release systems for fire prevention. It is an efficient fire extinguishing agent for Class B fires (flammable liquids and gases) and Class C (electrical) type fires. In medicine it is used to prevent or to treat a potassium deficiency called hypokalemia, an electrolyte replenisher. The compound is also an excipient which is an inactive substance made alongside the active ingredient of a medication. The reason is for bulking-up formulations that contain potent active ingredients. Potassium bicarbonate is in things like household odor remover and an accelerator in fast setting cements.[4]
Health Concerns
Potassium bicarbonate when used in medicine it may have some effects and concerns. Recently there have been some studies dealing with Potassium bicarbonate. These have shown that Potassium bicarbonate may improve bone health and the cardiovascular system. Some side effect to this compound are: confusion,uneven heartbeat,unusual tiredness, weakness, heavy feeling in your legs,severe stomach pain cramping, and black, bloody, or tarry stools. Less serious effects may be nausea, vomiting, diarrhea, upset stomach, a rash, slight tingling in the hands or feet, and anxiety. [5]
In fire extinguishers, Potassium bicarbonate is most commonly used because it does not give off many harmful side effects. It does not effect people and animals when sprayed from fire extinguishers.
Video
This explains more about Potassium bicarbonate in fire extinguishers.
References
- ↑ 1.0 1.1 Stocks, Bobby. Uses for Potassium Bicarbonate eHow. Web. Date of access 1/7/14.
- ↑ AMSPotassium Bicarbonate.web. 11/25/1999 Date of access
- ↑ MollenWhat Is Potassium Bicarbonate Used For?ehow.Web.1/3/2014 Date of Access
- ↑ POTASSIUM BICARBONATE FACTS, APPLICATIONS & OPPORTUNITIESArmand Products.Web.1/3/2014 Date of Access
- ↑ potassium bicarbonate Norris Cotton Cancer Center. Web. Date of access 1/22/2014(specify which).
| ||||||||||||||


