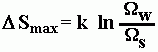Order from chaos
Order from chaos demonstrates in mathematical terms that a system can only become more ordered when the system is acted on by energy more ordered than the system itself. Conversely, a system decreases in order when it is acted on by energy less ordered than the system itself.
Random Mutations
From this it is inferred that "mutation and natural selection" are inherently incapable of increasing information and order in a system, because random mutations are less ordered than the genome itself, and therefore capable only of decreasing the order in the genome. Natural selection is merely a "quality control" mechanism whereby the worst mutations and those characteristics not suited to a particular environment are weeded out. However, neither mutation nor natural selection are capable of accounting for an increase in genetic order and information.
Entropy, disorder, and randomness
When entropy is examined statistically, it can be considered a measure of randomness. Now the more random a system is, the more disordered it is. The formula for statistical entropy is:
S is entropy.
k is the Boltzmann Constant = 1.380 6504(24) X 10-23 J K-1
![]() is the number of equivalent equally probable configurations. This is a direct measurement of disorder.
is the number of equivalent equally probable configurations. This is a direct measurement of disorder.
Random or disordered systems have such a significantly higher number of equivalent equally probable configurations, that they can basically be considered inevitable. Now entropy is not quite the same as disorder, but entropy is logarithmically related to disorder. Entropy can be considered a measurement of disorder in the way that the Richter Scale is a measurement of earthquakes or decibels are a measurement of sound. The result is that it is accurate to call entropy a measure of disorder. This means a reduction in entropy does result from an increase in organized complexity. wikipedia
Relation with the Second Law
While the following concept is related to the 2nd law of thermodynamics, it extends beyond the basic concept of the second law. It comes from a statistical analysis of how energy applied to a system affects entropy. It is evident from the difference between construction work and a bomb that how energy is applied is critical to an increase or decrease in entropy.
Consider a pile of wood. If a group of people work to organize the pile of wood, a building can be built that has less entropy than the pile of wood. If however a bomb of equal energy is applied to the pile of wood, the result is that the pile is scattered with more entropy than the pile of wood.
Now any time energy is applied to a system there is some degree of randomness in how it is applied. The result is that the randomness of the system is moved towards that of the applied energy.
![]() = The number of equivalent equally probable states of the system.
= The number of equivalent equally probable states of the system.
![]() = The number of equivalent equally probable states of the application of energy.
= The number of equivalent equally probable states of the application of energy.
Now the statistical formula for entropy is:
This results in:
and
The result is that:
or
Where:
![]() = Entropy of energy application.
= Entropy of energy application.
The result is that if energy is applied to a system in a manner more random than the system, then it becomes more random. If the same amount of energy is applied to a system in a manner less random than the system, then it becomes less random. This explains why organized work can build buildings, but a bomb will bring it down. The more organized application of energy would be an organizing force.
Natural Selection and Order
Evolutionists claim that natural selection communicates information from the environment to populations of organisms. In fact natural selection communicates nothing. All it is, is a quality control mechanism. Natural selection can only select from what already exists. In and of itself, natural selection causes no changes in DNA. The real source of genetic change for evolution is random changes in DNA called mutations.
Now DNA is very organized, while mutations are very random. This results in the following:
then
Plugging this in to formula 1:
This results in:
This means that random mutations can only increase the entropy of DNA. Furthermore since:
then
This means that mutations will increase the randomness of DNA. As a result the best that natural selection can do is to remove the most randomized DNA. So natural selection cannot communicate anything, therefore the general theory of evolution has no organizing force and no program. The result is that since the general theory of evolution needs to decrease the entropy of DNA and lacks an organizing force it would seem to contradict the laws of Thermodynamics.
Related References
- CAN ORDER COME OUT OF CHAOS? by Henry Morris and John Morris. BTG No. 102. 1997
- J Philip Bromberg, Physical Chemistry, 1984, pg. 690
See Also
| ||||||||||||||||||||