Methanol
| Methanol | |
|---|---|
| |
| General | |
| Systematic name | Methanol |
| Other names |
Carbinol |
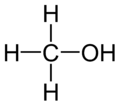
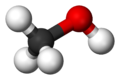
| Molecular formula | CH3OH or CH4O |
| Molar mass | 32.04 g mol−1 |
| Appearance | Colorless liquid |
| CAS number | 67-56-1 |
| Properties | |
| Density and phase | 0.792 g/cm |
| Solubility in water | miscible |
| Melting point | −97.6 °C (−143.7 °F; 175.6 K) |
| Boiling point | 64.7 °C (148.5 °F; 337.8 K) |
| Vapor pressure (pKa) | 13.02 kPa (at 20 °C) |
| Hazards | |
| MSDS | Methanol MSDS Page |
| Flash point | 11 to 12 °C (52 to 54 °F; 284 to 285 K) |
| Autoignition temperature | 470°C (878 °F; 743 K) |
| Explosive limits | 6–36% |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Methanol is the liquid chemical of the simplest chemical formula CH3OH, a series of organic compounds called alcohol, which is composed of a methyl group (CH3) linked to a hydroxyl group (OH). It is colorless, volatile, flammable, and toxic. Methanol is made from the destructive distillation of wood and is mainly composed of carbon monoxide and hydrogen. Its principal uses are in organic synthesis, as a fuel, solvent, and antifreeze. Methanol is a polar liquid at room temperature. Methanol is often called wood alcohol because it is it is primarily produced as a by product of the destruction distillation of wood.[1]Today, three methods are commonly used to produce synthesis gas from the methane component in natural gas. The method is steam methane or SMR, which is endothermic, partial oxidation with molecular oxygen, which is exothermic, and the combination of the two, which is referred to as autothermal reforming. [2]
Properties
Chemical
Methanol is produced in the anaerobic metabolism of many types of bacteria. There is a small portion of the methanol vapor in the atmosphere. Atmospheric Methanol gets sunlight and is oxidized by oxygen to carbon dioxide and water. Methanol is burned in air to form carbon dioxide and water. At room temperature it is a polar liquid and is used as a denaturation for antifreeze, solvent, fuel and ethyl alcohol. It is also used to produce bio-diesel through transesterification reactions. Methanol flame is almost colorless, causing an additional safety hazard around open methanol flames.[3]
Physical
Methanol is the simplest of the series of organic compounds called alcohols. The molecular formula is CH3OH. A modern method of producing methanol is based on the direct combination of carbon monoxide gas and hydrogen in the presence of a catalyst at elevated temperatures and elevated pressures. Most of the Methanol is produced from methane in natural gas. Pure methanol is an important chemical compound. Its derivatives are used in large amounts to make numerous compounds from a number of important synthetic dyes, resins, drugs and perfumes. In the case of synthetic resins, dyes are converted to formaldehyde and larger amounts are converted to dim-ethyl aniline. It is also used for automotive antifreeze, rocket fuel and general purpose. And it is also a high octane, clean combustion fuel that can replace petrol in cars.[4]
Synthesis
The syngas is catalytically converted to methanol in the fixed bed reactor at high temperature and high pressure. The catalyst is alumina pellets coated with copper and zinc oxide. Considering the energy of the reaction, it can be seen that the yield of methanol is favored by high pressure and low temperature. The low pressure process consisted of the discovery of copper based catalysts that were active at 475-575 K, enabling economical conversion at 40-100 atmospheres. For example, one plant operates at 525-575 K and 100 atmospheres. Eventually we achieve 97% conversion of the reactants. The actual mechanism for Methanol formation was an active area of research. By using radioactive 14CO2, it is believed that most, if not all, of the methanol is induced through CO2.[5]
Uses
Methanol is essential for our daily lives. Until we woke up, we were able to perform routine tasks with methanol. Methanol is found in silicon entering the shampoo. Pets make plastic bottles bought at convenience stores. We can find Methanol in many components of the car you drive and find fuel used in our car. It also exists in medicines to help fight against colds and fleece jackets that are worn to wear warm clothes. And it is often used as a modifying additive for industrial ethanol because it is toxic. It is often called wood alcohol because it is principally produced as a by product of the destruction distillation of wood. Today, three methods are commonly used to produce syn-gas from methane in natural gas. This method is steam methane or SMR, which is endothermic, partial oxidation with molecular oxygen, exothermic reaction, also known as auto thermal reforming. It is now produced synthetically by a multi step process, natural gas and steam are reformed in a furnace to produce hydrogen and carbon monoxide. Then hydrogen and carbon monoxide gases react under pressure in the presence of a catalyst.[6]
History
Ancient Egyptians used a mixture of substances that included methanol in their embalming process. They obtained the methanol from pyrolysis of wood. Pyrolysis is the chemical disintegration of condensed organic substances by heating. However, pure Methanol wasn’t isolated until 1661 by Robert Boyle, who produced the chemical through the distillation of boxwood. The chemical later became known as pyroxylic spirit. The French chemists Jean-Baptiste Dumas and Eugene Peligot determined its elemental composition in 1834. The term “Methyl” was derived from the word “Methylene,” which was coined by Dumas and Peligot in 1840. It was then applied to describe “methyl alcohol.” The International Conference on Chemical Nomenclature shortened this to “Methanol” in 1892. On January 12, 1926, German chemists Alwin Mittasch and Mathias Pier developed a means to convert synthesis gas into methanol, a patent was filed.[7]
Video
Methanol Vs. Ethanol
References
- ↑ Methanol chemical compound ENCYCLOPÆDIA BRITANNICA.2018. Unknown
- ↑ Methanol The chemical company. 2018. Unknown
- ↑ Chemical and Physical Properties of the Methanol Molecule world of molecules. March 5, 2018. Unknown.
- ↑ PHYSICAL PROPERTIES OF METHANOL: CH3OH cetiner engineering corporation. March 2, 2018. Unknown
- ↑ Methanol the essential chemical industry-online.February, 17 2017. Unknown
- ↑ Methanol is Everywhere MGC METHANOL. March 5, 2018. Unknown
- ↑ Methanol The chemical company. 2018. Unknown
| ||||||||||||||


