Methane
| Methane | |
|---|---|
| |
| General | |
| Other names | Marsh gas and Firedamp |
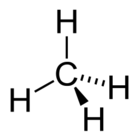
| Molecular formula | CH4 |
| SMILES | C |
| Molar mass | Molar mass::16.0425 g/mol |
| Appearance | colorless gas |
| CAS number | CAS number::74-82-8 |
| Properties | |
| Density and phase | Density::0.717 kg/m³, gas |
| Solubility in water | 3.5 mg/100 ml (17 °C) |
| Melting point | Melting point::−182.5 °C |
| Boiling point | Boiling point::−161.6 °C |
| Structure | |
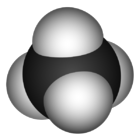
| Molecular shape | Tetrahedral |
| Hazards | |
| Main hazards | Highly flammable (F+) |
| MSDS | External MSDS Data |
| NFPA 704 | |
The chemical methane is a colorless and odorless gas that is lighter than air[1] and it comes from both human-related sources and natural sources[2]. Methane has many different uses and it can also be a hazard because of it's flammable properties.
Properties
Methane is a gas that is not only lighter than air, but it is also both colorless and odorless. Methane can be formed when organic carbons that are under anaerobic conditions decompose and because of this, it is usually found in wetland areas, swamps, wood-wastes, landfills, and peat deposits. There are many different factors which determine the rate of methane production. Some of these factors are how much rain falls on the organic matter, the type of the organic materials that are producing the Methane, and also, the temperature.
Some other chemical properties of Methane is that it can be renewed because it comes from the decaying of organic material. Methane can also be dissolved in alcohol, carbon tetrachloride, and other non-polar solvents. The melting point of this chemical is -182.5 °C and because it is a non-polar molecule, it can not be dissolved in water. When burned, methane gives off a blue flame and it burns to give off carbon dioxide and water. It is used as a fuel because it gives off a lot of heat. [3]
Occurrences
Methane is emitted from several different sources. Some are human-related (anthropogenic) and others come from natural sources. Some natural sources that produce methane are gas hydrates, freshwater bodies, non-wetland soils, oceans, wetlands, termites, permafrost, and other sources like wildfires. The human-related (anthropogenic) sources/activities are things such as biomass burning, waste management, fossil fuel production, rice cultivation, and animal husbandry (manure management and enteric fermentation in livestock). All of these procedures release quite a bit of methane into our atmosphere. It has been estimated that globally, 60% of the methane emissions are from or are related to human-related activities.
The emission levels for methane from a source can vary a lot from different countries and regions. The emission levels depend on different factors like climate, energy types and usage, waste management practices, temperature, moisture, and industrial and agricultural production. The temperature and moisture in an area can effect the anaerobic digestion process, which is one of the main biological processes that create methane emissions in human-related sources and natural sources. Technology used for getting methane from coal mines, landfills, and manure management systems is also able to effect the emission levels that are coming from these sources.[4]
Uses
There are both fuel and industrial uses of methane. Methane is used as a fuel in electrical generation and its burned in a gas turbine or in a steam boiler. Methane when burned produces less carbon dioxide than other hydrocarbon fuels and the heat of combustion for methane is around 802 kJ/mol, and that is also lower than other hydrocarbons. Not only that, but out of all the other complex hydrocarbons, methane produces the most heat per unit mass. Methane is also used for domestic heating and cooking in homes.
Methane is also used to fuel vehicles when it is compressed natural gas and is better for the environment than diesel and gasoline/petrol. NASA is also doing research to find out if methane will also be a good fuel for rockets. This will be helpful because methane can be found in many different places in the solar system and so if need be, it can be harvested for a return trip to earth.[5]
Health Effects
Methane is a very flammable chemical and even though it is not toxic, it can form an explosive mixture with air when in a confined area. The explosive range of this chemical is between 5% and 15% methane-in-air.[6] This chemical is reactive with halogens, oxidizers, and some halogen-containing compounds and can cause violent results. A risk for buildings built near or on landfills is having methane off-gas coming into the inside of the building and then exposing the people and animals inside to high levels of methane. A few buildings like the Dakin Building in Brisbane, California have specially engineered a system in their basements to catch this off-gas and vent it so that it does not come into the building.[7]
Methane In Our Solar System
The following locations are where Methane is believed to exist in our solar system or has been detected:
- Jupiter
- Pluto: Charon
- Neptune: Triton
- Eris
- Uranus: Ariel, Umbriel, Titania, Miranda, Oberon
- Mars
- Saturn: Titan, Enceladus, Iapetus
- Comet Halley
- Comet Hyakutake
References
- Methane Wikipedia
- City of Burnaby
- Methane U.S. Environmental Protection Agency
| ||||||||||||||



