Basement membrane
The Basement Membrane is a thin sheet of fibers that underlies the epithelium, which lines the cavities and surfaces of organs including skin, or the endothelium, which lines the interior surface of blood vessels. The membrane is composed of an important group of molecules that lie outside of the cell in what is called the extracellular matrix (ECM).
These molecules were long ignored, but as it turns out that the ECM molecules are vitally important to cells. ECM molecules bind to receptor molecules on the surface of the cell and send signals that regulate which genes are active in the cell.
Laminin
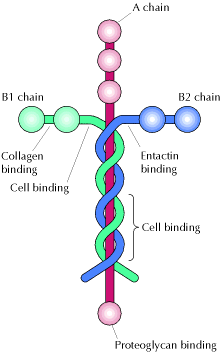
One of the major ECM molecules is laminin, a large cross-shaped protein. Laminin is the extracellular "glue" that holds out tissues together.
The laminins are a family of extracellular matrix glycoproteins localized in the basement membrane that separates epithelial cells from the underlying somatic tissues. They are also found in basement membrane surrounding fat, muscle and peripheral nerve cells. The laminins are the primary extracellular matrix molecules to be observed in the developing embryo and in mature adult differentiated tissues.
In addition, there have been new developments in the number and localization of the homologues of the laminin chains and the role of laminin in neuromuscular disease. Their primary role is in cell-matrix attachment, but many additional biological activities, including promoting cell growth and migration, tumour growth and metastases, neuron outgrowth, nerve regeneration, wound repair and graft survival, have been demonstrated. Many of these biological activities are duplicated by proteolytic fragments of laminin and by small laminin-derived synthetic peptides. These laminin-derived peptides may be useful clinical reagents for accelerating wound healing with minimal scarring or for blocking tumors.
Structure and Nomenclature
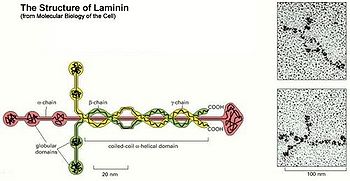
The laminins are large glycoproteins comprising three different disulphide-bonded chains. Laminins are incredibly important component of our connective tissues, contained mostly in the basement membranes throughout the human body and all animals. Laminin nomenclature is based on which of the 3 polypeptide chains the molecule contains. For example, Laminin-521 has the #5 alpha helix, the #2 beta helix and the #1 gamma helix comprising the three-part chain structure. The laminins are the primary extracellular matrix molecules to be observed in the developing embryo and have potent biological activities. In addition, there have been new developments in the number and localization of the homologues of the laminin chains and the role of laminin in neuromuscular disease. Their primary role is in cell-matrix attachment, but many additional biological activities, including promoting cell growth and migration, tumor growth and metastases(cancer growth), neuron outgrowth, nerve regeneration, wound repair and graft survival, have been demonstrated. Many of these biological activities are duplicated by proteolytic fragments of laminin and by small laminin-derived synthetic peptides. These laminin-derived peptides may be useful clinical reagents for accelerating wound healing with minimal scarring or for blocking tumors. The laminins include fifteen identified varieties of cross-shaped glycoproteins making up the extracellular matrix that connects to collagen, providing a molecular framework supporting tissues and determining how healing takes place in some organ systems such as the CNS and kidneys.
According to various sources, laminin (especially laminin-532) is involved in intracellular communication and even in clotting as well as healing. The expression of laminin production is controlled by growth hormone and laminins are involved in directing cells to the correct place during the body's response to injury. One of laminin's main functions is to help epithelial cells in basement membranes, including blood vessels, to communicate with other cells and to know where to move during developmental stages. On the top of the cross shaped laminin molecule is the N-terminal domain which is important for the binding of laminin together with other matrix molecules.[1] For actual sequencing data for the laminin protein isoforms, there is an online database that lists data for human laminin.[2]
Structure and Function
There are about 15 known different types or isomers of laminin glycoproteins that consists of one alpha, one beta, and one gamma protein chains, making a trimeric complex of strands encircling a cross shaped sugar backbone. There may be more isoforms existent in mammalian tissues but since isolating most of the laminins from the rest of the extracellular matrix is so difficult, progress has been slow.[3] These laminin glycoproteins are classified according to which polypeptide chains are included. For example, the laminin-5 molecule was renamed laminin-332 and laminin 10 was renamed laminin-511 that contains one of five alpha chains, one of three beta chains, and one of three possible gamma chains. These two particular laminins are critical to the structure of the laminar basement membrane of skin tissue between the epidermal dermal layers. The portion of the laminin molecule that binds laminar membrane together is the globular LG domain at the bottom of the cross. The LG domain is the C-terminal portion of the alpha helix with five different defined subdomains. Since the variety of five possible alpha helices varies the shade of the LG domain, C-terminal ends very among the laminins. There are also a variety of membrane receptors that bind LG domain of laminins: syndacans, a-dystroglycan, and integrins. Dystroglycan is a plasma membrane glycoprotein that normally tightly binds to laminin. In many muscular dystrophies and epithelial cell cancer, glycosylation (addition of sugar side chains) on one of the polypeptide chains is disrupted. Injection of purified laminin-111 in mice restores muscle regeneration by alpha 7 integrins and their involvement in binding laminin tightly. This research finding may lead to a new treatment for some types of muscular dystrophy, especially alpha 7 integrin congenital myopathy.[4]
Experiments with a nude (hairless) mouse phenotype that lacks the LAMA gene that codes for laminin-511 that is critical for mouse development, specifically for the growth of hair. Not only are these mice hairless they also suffer development problems in the placenta (placentopathy), and die within day 17 of their fetal development. Normal laminin -511 expression has been shown to be critical for hair development since studies using antibodies to laminin-511 inhibit follicle morphogenesis. Laminin- 511 is also important to the regulation of hair growth, especially in newborns but its loss was also implicated in age related hair loss. Special mutant phenotypes of mice affected by point, frame-shift, and nonsense mutations in the LAMA genes experience severe epidermal blistering since the lack of laminin-332 prevents the adhesion between membrane surfaces. Humans that have a mutation in laminin-332 genes (at least 3 different LAMA genes), on the other hand, suffer a condition called junctional epidermolysis bulossa (or JEB) also characterized by severe skin layer separation and blistering. This is caused by the destabilization in the extracellular matrix between the epidermis and the dermis due to a mutation in the LAMA3 gene and an absence of mRNA coding for laminin-332. Since integrin receptors are also essential to the signaling action of laminins in the basement membrane, any mutation in these receptors causes both serious skin and hair problems. Since most anticancer drugs affect the integrin receptors in hair follicles, this leads to hair loss during chemotherapy. Some studies have even lent support to the possibility that laminin-332 Binding to integrin receptors may regulate the expression of other signaling proteins and kinases such as Src kinase and MAP kinase. Epithelial cell proliferation may be mediated by the interaction of laminin 332 with the MAP kinase pathway, as well as interaction with epithelial growth factor (EGF). Although laminin-332 undergoes proteolytic activation after secretion, laminin-511 is secreted in active form and incorporated immediately in the membrane between the epidermis and the dermis layer throughout the integumentary system.[1]
Role in Adhesion and Wound Healing
Keratinocytes that produce the skin protein keratin are critical not only to the skin’s integrity but also to the regeneration of keratin layers in wound repair and nail replacement. The interaction between beta1 integrin receptors and laminin-332 has been shown to be essential to keratin repair and survival. Structures called hemidesmosomes (desmosomes being structures connecting epithelial cells to the basement membrane, BM) have been likened to spot welds connecting each epithelial cell and keratinocyte to the laminin molecules in the basement membrane. This strong adhesion of cells to the BM is also reinforced by the collagen protein BP 180. Altogether the result is an extracellular matrix proteins, similar to the rebar that reinforces cement, that includes firm attachments between the laminar proteins, the BM, and cell layers. It is no surprise that both laminins 332 and 511 (as well as some others) are critical to the process of wound healing and cell migration [8]. The molecular mechanisms that mediate the communication between laminin-receptor complexes and cell junctions remain unclear and yet such signaling may be a crucial event during wound healing and the control and dissemination of tumor cells. Laminin-511 and -332 both play important structural roles in the BM of the skin. The importance of laminin-332 is emphasized by diseases in which laminin-332 is either the target of pathogenic autoantibodies or is subjected to mutation, resulting in a blistering phenotype. However, these laminins also play key roles as regulators of keratinocyte migration in wound healing, tumor genesis, and modulate skin appendage development. They do so by interacting with various integrin cell surface receptors, through which they regulate specific signaling pathways.[5]
Role in the insulation and protection of nerve cells
Studies in cell culture have suggested that laminin plays a critical role in myelination. In vivo studies have also suggested that laminin is important. Mutations in the laminin alpha-2 gene in mice result in various types of muscular dystrophy. Because the mutations also affect the muscle, the phenotype is a combination of effects on the nerve and the muscle. Other evidence suggesting a possible role for laminin in myelination stems from the observation that ß1 integrin, a component of many laminin receptors, is necessary for proper myelination of peripheral nerves. Laminin-2 is involved in the formation of myelin sheaths wrapped around every axon. This glycoprotein insulation accelerates the conduction of the nerve impulse from one end of the cell axon to the other.
In earlier studies, laminin expression was always associated with Schwann cells. Schwann cells are known to be a major source of laminin in nerves, especially during development. In earlier developmental stages before laminin- gamma-1(abbrev. Laminin- g1) its expression is critical to myelination. When unmyelinated axons are checked for laminin, only nascent laminin is found in situ. Chen and Strickland (2008) created mice that were homozygous for the fLAM1 allele that also carried a transgene with the calcium/calmodulin-dependent protein kinase II (CaMKII) promoter, driving expression of the Cre recombinase. Mice of this genotype (CaMKII/Cre:fLAM1, referred to as mutant mice) exhibited hind leg paralysis and tremor. Analysis of these mice revealed significant recombination in the hippocampus, spinal cord, and peripheral nerves (e.g., the sciatic nerve), but little to no recombination in other organs such as the muscle or heart, meaning the expression of laminin-g1 activated by this gene promoter (CaMKII) is specific to nerve cells. Finally, when the laminin-g1 gene was disrupted in Schwann cells using Cre driven by the P0 promoter, a promoter specific for Schwann cells, there was also no laminin-g1 staining in the axons. These results demonstrate that Schwann cells are the major source of laminin in the nerves and that reduced expression of laminin-g1 in the mutant nerves is due to recombination in Schwann cells.[5]
Role in Controlling Cancer
In many types of cancer, metastasis (systemic spreading) of cancer cells involves lack of adhesion to laminin proteins and integrin receptors that normally control movement of cells through a process called anchorage dependence. Spreading cancer cells are no longer anchored to the ECM and become dangerous liabilities to normal tissue.[6]
- As molecular biologists point out, tissues are cellular communities that rely on the self-sacrifice of its members rather than “survival of the fittest”. Laminin mediates cellular communication that enables cellular unity.[6]
- Cancer cells are the obvious exception to this biological rule, due to their spread and growth
at the expense of normal cells.
- Normal cells respond to extracellular signals from the basal lamina as well as other cells while cancer cells lose the ability to bind signaling and transducing proteins.[6]
- Many of the differences between cancer cells and normal cells are due to modifications made to new cells by the process of glycosylation, as well as addition of other chemical side chains.[7]
- Epithelial cell cancers are often caused by a lack of proper addition of polysaccharide side chains to dystroglycan on epithelial cell membranes.[7]
References
- ↑ 1.0 1.1 Sugawara et al. 2008. Laminin-332 and -511 in skin. Experimental Dermatology, 17: 473–480.
- ↑ The Laminin Protein Sequence Alignment of the Laminin Protein (Homo sapiens) (with Clustalw), Author of database not listed.
- ↑ Colognato, H. and P. Yurchenco. 2000. Form and function: The laminin family of heterotrimers Developmental Dynamics, Volume 218 (2): 213-234.
- ↑ Potential Therapy For Congenital Muscular Dystrophy Am J Pathol, 174: 256-264. ScienceDaily, January 1 2009. Accessed August 24 2010.
- ↑ 5.0 5.1 Chen, Zu-Lin and S. Strickland. 2008. Laminin 1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. The Journal of Cell Biology, Volume 163, Number 4, 889-899.
- ↑ 6.0 6.1 6.2 Alberts et al. 2008. Molecular Biology of the Cell, 5th edition. New York: Garland Science, pp 1058-1205.
- ↑ 7.0 7.1 Unusual Protein Modification Involved in Muscular Dystrophy, Cancer University of Iowa. ScienceDaily, Jan. 3, 2010.