Isopropyl alcohol
| Isopropyl alcohol | |
|---|---|
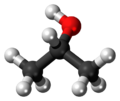 
| |
| General | |
| Systematic name | 2-propanol |
| Other names |
Isopropanol |
| Molecular formula | (CH3)2CHOH |
| SMILES | CC(C)O |
| Molar mass | Molar mass::60.1 g/mol |
| Appearance | Clear liquid |
| CAS number | [[CAS number::67-63-0
]] |
| Properties | |
| Density and phase | Density::0.785 g/ml, Liquid |
| Solubility in water | Miscible with |
| Melting point | Melting point::-88°C |
| Boiling point | Boiling point::82°C |
| Acidity (pKa) | 16.5 |
| Viscosity | 0.00243 cP at 20°C |
| Structure | |
| Dipole moment | 1.66 D |
| Hazards | |
| MSDS | Material safety data sheet |
| Main hazards | Flammable |
| NFPA 704 | |
| Flash point | 11.7°C |
| R/S statement | R: R11, R36, R 67 S: S7, S16, S24, S25, S26|- |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Isopropyl Alcohol, more commonly known as rubbing alcohol, is found in many homes and medicine cabinets. This chemical compound has no color but a strong bitter and alcohol-like odor. Its wide range of uses and low cost makes it easily found and readily available. Businesses, housekeepers, nurses and those who work with computers use this product to keep things sterilized and to gently clean messes. While Isopropyl Alcohol can become dangerous if used in large amounts or digest, the usefulness of it keeps it a popular product.
Properties
Isopropyl alcohol is a colorless liquid with the scent of ethanol or acetone and a strong bitter taste. It is flammable when in liquid state, but not in gaseous form. It is very dangerous to aquatic life, fish have been known to die when large quantities of Isopropyl Alcohol are poured down sewers or sinks. [1] In solutions of salt it is insoluble, though soluble in acetone, benzene and chloroform. Isopropyl Alcohol will react against certain forms of plastic and rubber. The burning of it may lead to the release of carbon monoxide into the atmosphere and contact with fire or heat can cause isopropyl alcohol to explode. isopropyl Alcohol should be stored in a dry place with a temperature between 5 and 30o Celsius [2]
Synthesis
The simple way of explaining process of creating Isopropyl alcohol is by joining water and propane. To complete this process you can either hydrate the propane directly or indirectly with sulfuric acid. Distillation is required to separate the isopropyl alcohol from the water. [3]
Direct Hydration
Direct Hydration is most widely used in European countries. This method uses an acid based catalyst to speed the process. The propane is used in either gas or liquid phases. heat and pressure must be heavily applied.
Indirect hydration
The upside for using the indirect hydration process is that a cheaper version of propane; this is the way the USA created is Isopropyl alcohol. The propane and sulfuric acid are mixed together, created esters of sulfur. The esters are then joined with water and put through a hydrolyzer machine.
[4]
Isopropyl Alcohol occurs in cognac and apples. [5]
Uses
Isopropyl alcohol has many uses, both in households and labs. It is a polar solvent-meaning it dissolves other polar compounds. It can be used as a process solvent which extracts and purifies oils, fats, vegetables, waxes and more. It is often used in detergents, soaps and glass cleaners as the main cleaning agent. The most known use for Isopropyl alcohol would be the inexpensive and easily acquired rubbing alcohol, used on skin for minor cuts or in preparation for injection. If put on skin for two minutes it will cut the bacteria count by five percent. As an antiseptic, it fights vegetative bacteria.[6] It is also a popular electronic cleaner; it can be used along with a piece of cotton to polish cell phones, computers, and cameras. In addition, it is popular for removing stickers from merchandise. Isopropyl alcohol is sometimes used on hands to remove sticky residue and strong smells. isopropyl Alcohol is unique in that while it can remove sticky or gooey substances, it is also used in the manufacturing of several of them, including nitrocellulose film-a flexible film used for photography. It can be a replacement for massage oils, because of its muscle soothing heat and power. This compound has the power to kill fleas and ticks off of pets as well as furiture. When making an ice pack, many people add Isopropyl alcohol to keep the water from freezing solid. When formaldehyde is not readily available, or cannot be used, isopropyl alcohol can take it's place. This compound is also often added to gases because it renders the water in a tank soluble. When scientists need to extract chromosomes isopropyl alcohol can be used along with detergents. Artists may add this to their water colors while pet-lovers use it to scrub cages. It is often used for office cleaning jobs as well-such as removing permanent marker. For cosmetic purposes it is useful for removing acne and creating eyeshadow.[7]
DNA is insoluble in Isopropyl alcohol so it is often extracted by using this compound. [8] This alcohol is also useful in the curing of swimmers ear. It dries the residual water left in an ear. [9]
Poison
Isopropoyl Alcohol is toxic if ingested or with chronic exposure to skin. Once ingested this alcohol's effects begin in 30 minutes and complete their cycle in 120. Cramps, dizziness, and vomiting are common effects from the alcohol. Children most commonly are victims of this household object, often found in bathroom cabinets. When large amounts are inhaled, lightheadedness and nauseousness occur quickly. If left on skin for over 4 hours serious nerve damage could occur, and eventually even eczema can develop. Irritation occurs when in contact with eyes.[1] Those working in close contact with Isopropyl Alcohol are advised to get routine tests for chronic poisoning. After coming in contact with this substance workers are advised to wash with soap and water and change clothing. Isopropyl alcohol is a popular poison among children because of the easy access to it. Once the liver has given the isopropyl alcohol oxygen it becomes acetone, which attacks your central nervous system. Cases of purposeful poisoning with antifreeze-often made with isopropyl alcohol-is commonly heard of as well. Unsuccessful poisoning attempts or accidental swallowing often require the stomach to be pumped.[10]
References
- ↑ 1.0 1.1 Isopropyl Dr Peter Hayes , Publisher, Date.
- ↑ Occupational Safety and Health Guideline for Isopropyl Alcohol Author Unknown, Accessed 2-11, United States Department of Labor
- ↑ Isopropyl Alcohol Author Unknown, Accessed 2-16, AbsoluteAstronomy
- ↑ How Is Isopropyl Alcohol Made? LaTasha Favors Accessed 2-15, EHow
- ↑ isopropyl alcohol Author Unknown, Accessed 2-11, The Good Scents Company
- ↑ Isopropanol Author Unknown, Chemical Land 21, Accessed 2/11.
- ↑ Uses for Isopropyl Alcohol Denise White, October 2008, Associated Content
- ↑ Isopropyl Alcohol: The Super Hero of the Tech Cleaning World Sarah Rae Trover, August 2010 , Unplgged
- ↑ Isopropyl Alcohol Poisoning - When Too Much of Anything Can Be Damaging Jo Alesto, May 2009, EzineArticles
- ↑ The Effects of Consuming Rubbing Alcohol Melinda Dean, Accessed February 2011, EHow
| ||||||||||||||



