Chloroacetophenone
| Chloroacetophenone | |
|---|---|
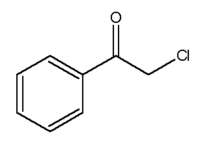
| |
| General | |
| Systematic name | Chloroacetophenone Phenacyl chloride |
| Other names |
2-Chloro-1-phenylethanone |
| Molecular formula | C6H5COCH2Cl |
| SMILES | c1ccc(cc1)C(=O)CCl |
| Molar mass | Molar mass::154.59 g/mol |
| Appearance | White Crystalline Solid |
| CAS number | CAS number::532-27-4 |
| Properties | |
| Density and phase | [[Density::1.324 g/cm3]] solid |
| Solubility in water | Insoluble in Water; Soluble in Alcohol, Benzene, and Ether |
| Melting point | Melting point::58°C |
| Boiling point | Boiling point::247°C |
| Structure | |
| Molecular shape | Tetrahedral |
| Crystal structure | Hexagonal |
| Hazards | |
| MSDS | Material safety data sheet |
| Main hazards | Explosive, flammable, and toxic through oral ingestion; irritant to skin and eyes |
| NFPA 704 | |
| Flash point | 118°C (244°F) |
| RTECS number | AM6300000 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Chloroacetophenone is a chemical compound that is mainly used in the production of tear gas and chemical mace. It exists naturally in a solid form, but can be converted into an aerosol agent. In this form, it is used by law enforcement to keep order in chaotic and out-of-control situations. However, the use is not without its consequences, and the effect that it can have on an individual can be very dangerous.
Properties
Chloroacetophenone in its purest form appears as a white crystalline solid. Its size is similar to granulated sugar and salt, and bears a distinct similarity in appearance to both the element and the carbohydrate. To be used as an irritation agent, it must projected as a very fine dust, or be carried through the air as part of an aerosol irritant.[1] [2]
The effects of chloroacetophenone on humans include lacrimation (tearing), irritation of the eyes, photophobia (an extreme sensitivity to light), and a burning sensation on the surface of the skin. [1][3] However, if an individual is under the influence of narcotics or alcohol to the extremity that their tactile senses have been lost, chloroacetophenone's usual effects on their body are virtually nonexistent. Also, the effect of chloroacetophenone on certain animals is virtually nothing. Tests have shown that a dog bound in a fixed position with little or no movement will not make very significant, if any, attempts to avoid chloroacetophenone as an aerosol agent.[1] However, tests conducted on guinea pigs, mice, rabbits, and rats showed an extremely toxic reaction from oral exposure.[3]
Decontamination
In order to decontaminate oneself after being exposed to chloroacetophenone, a soda ash solution or alcoholic caustic soda must be applied to the affected area. The individual that has been exposed should also be taken outside into the fresh air, avoid rubbing their eyes or face, and immediately wash their eyes and/or face. Soaps that contain oil should not be used in the cleansing process due to a property in oil that will trap the chloroacetophenone, causing a severe rash or burn. Clothes that have any residue from the chloroacetophenone spray should also be changed.[1]
Hazards and Dangers

There can be several dangers and health hazards that come from dealing with chloroacetophenone, including those of a chemical nature. In a reaction when chloroacetophenone is combined with strong oxidants, it will not be compatible. It does have a reaction with metallic substances that is slow, which causes corrosion in the metal. When coming into contact with the metal, there is a danger of the formation of a flammable hydrogen gas.[2]
Heating chloroacetophenone has the potential to form explosive mixtures with the different gases present in the air. This can be hazardous indoors and outdoors, especially if it is inside of a container. When burned, chloroacetophenone decomposes into a form that produces toxic and corrosive fumes, such as hydrogen chloride. Chloroacetophenone is combustible, so those using it should make certain that they keep it clear of anything that will ignite it. If ignited it will burn, but it does not ignite as easily as substances such as gasoline. Chloroacetophenone can also be converted into a molten form. If a small amount of chloroacetophenone is ignited, the best method for putting out the fire is to use an applicable form of carbon dioxide, which is an extremely dry chemical compound. It can also be extinguished using water spray of vapor. When a larger amount of chloroacetophenone is ignited, the two previously mentioned agents will extinguish it, along with an alcohol resistant foam.[2]
Symptoms and Duration
When an individual is exposed to chloroacetophenone, there are several different symptoms to indicate the contamination. General symptoms include:
- Instantaneous irritation of the individual's eyes, skin, and respiratory tract. Once decontaminated, the initial irritation should dissipate between fifteen minutes to half an hour. The irritation of the eye and the tissues surrounding may take one to two days to cease.
- An accumulation of fluid, known as pulmonary edema, can result from exposure to chloroacetophenone. This tends to occur immediately after the first exposure, but can be delayed as long as twenty-four hours.
- Detrimental effects on the skin which occur within twenty-four hours of exposure that include severe blistering, redness, and varying amounts of skin loss.
More specifically, symptoms are located on mainly four different areas on the body:
- The eyes: When the naked eye is exposed to a threshold concentration of chloroacetophenone, the symptoms are immediate pain, along with some blepharospasm (rapid and spasmodic blinking), lacrimation (the production of tears), and rhinorrhea (a runny nose). Other more generic symptoms of eye exposure include coughing, sneezing, and a severe redness of the eye. A more extensive amount of chloroacetophenone eye exposure results in conjunctivitis (the inflammation of the conjuctiva), corneal epithelium (the loss of the outermost layer of the cornea), keratitis (the inflammation of the cornea), photophobia (sensitivity to light), blurred vision, and chemical burns. Droplets and/or particles of chloroacetophenone that are directly in contact with the eyes can be be extremely corrosive and produce extreme burns, like those of a strong and powerful acid, and may also result in a loss of vision.
- The skin: Skin that comes into contact with mild or moderate amounts of chloroacetophenone will experience symptoms of irritation and pain. Moisture that that comes into contact with the area of exposure will most likely increase the irritation. A larger amount of exposure will cause erythema (redness), vesication (blistering), and areas of denuded skin.
- Ingestion exposure: Ingesting chloroacetophenone can be extremely discomforting. Symptoms include pain and discomfort in the epigastric (abdominal) region, burping, and a metallic taste with a burning sensation in the mouth. The chloroacetophenone is rather unlikely to get ingested, and will normally pass directly through the digestive system, unless it is regurgitated.
- Inhalation exposure: Milder amounts of inhaled chloroacetophenone will also cause rhinorrhea, as with eye exposure. It also results in coughing, sneezing, a tightness of the chest, vocal cord spasms, which may cause difficulties with breathing, a shortness of breath, a feeling of choking, a burning sensation along with excessive pain in the nose and mouth accompanied by a metallic taste in the mouth. Wheezing, salivation, nausea, and emesis (vomiting) may also occur. A more severe and extensive amount of inhaled chloroacetophenone may cause bronchopneumonia (the inflammation and consolidation of airspaces within the lungs), bronchospasm (narrowing of large airways), and pulmonary edema. Fear, pain, and panic in the exposed individual may cause hypertension (an elevated blood pressure), syncope (fainting), and tachycardia (an increased heart rate).[2]
Uses
Chloroacetophenone serves as the active ingredient of the aerosol irritant Chemical Mace, which became a standard piece of equipment for U.S. law enforcement agencies in 1965.[1][3] Those who use chloroacetophenone in the form of tear gas should wear an extensive amount of protective equipment in order to keep themselves from getting contaminated. Such protection as recommended by the National Institute for Occupational Safety and Health (NIOSH) includes:
- A CBRN full-face-piece SCBA that is operated in a pressure-demand method or a pressure-demand supplied air hose respirator with an auxiliary escape bottle to protect the user's face.
- A Totally-Encapsulating Chemical Protective (TECP) suit, providing maximum protection to the user's body against CBRN agents.
- Chemical-resistant gloves (outer) to protect the user's hands.
- Chemical-resistant gloves (inner) to provide maximum protection for the user's hands against any chemicals that get past the outer gloves.
- Chemical-resistant boots with a steel toe and shank to protect the user's feet.
- Coveralls, long underwear, and a hard hat worn under the TECP suit are optional items of clothing, but can provide the user with extra protection in case other equipment fails.[2]
Dissemination
The most common method for disseminating chloroacetophenone is in the form of a liquid spray, smoke, or vapor in both the indoors and outdoors. However, chloroacetophenone can also be used to contaminate food and water. If it released in an aerosol form near agricultural products, it can have the potential to make those products unfit for consumption.[2]
History
Chloroacetophenone was discovered by German scientists around 1870, but was not prepared for actual scientific use until 1877. The extent to which scientists used it was rather minimal, considering that its properties had not yet been explored by the scientific realm. However, in the year of 1923, the United States Government made the decision to finance the development of chemical agents that could be used for law enforcement at the Edgewood Arsenal. Scientists spent a great deal of time and energy researching irritants that would not be lethal to civilians but could be used as a method of crowd control. They eventually developed the chloroacetophenone as a spray in canisters that is very similar to the tear gas used by modern day law enforcement. In the late 1920's, the French became the first successful users of chloroacetophenone as a means of bringing order to the disputes of civilians within their colonies. By the 1930's, virtually every law enforcement agency in the world was using chloroacetophenone as a way of controlling out of hand civilian situations.[1]
Experimentation with chloroacetophenone continued into the 1940's during World War II. Within that time period, it was discovered that chloroacetophenone particles that were micro-pulverized would produce a greater effect of irritation that would endure much longer.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 CN Chloroacetophenone (CN) C6H5COCH2Cl. Zarc.com. Visited April 27, 2011.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 CHLOROACETOPHENONE (CN) :: Riot Control/Tear Agent. www.CDC.gov/NIOSH. Page last updated on August 22, 2008.
- ↑ 3.0 3.1 3.2 2-Chloroacetophenone. EPA.gov/TTN. Created in April 1992, Revised in January 2000.
Additional Information
- 2-Chloroacetophenone(532-27-4) ChemicalBook.com, Copyright © 2008
- CHLOROACETOPHENONE cameochemicals.noaa.gov, June 1999
- Chloroacetophenone www.chemyq.com
| ||||||||||||||


