Borate
| Borate | |
|---|---|
| |
| General | |
| Systematic name | Borate |
| Other names | Borate
Tetrahedral boron anions |
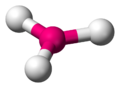
| Molecular formula | BO33− |
| Molar mass | Molar mass::58.811 |
| Monoisotopic mass | 58.995697 Da |
| CAS number |
CAS number::11129-12-7 14213-97-9 |
| Related compounds | Boric acid
Sodium perborate Borax |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Disclaimer and references | |
Borate is a polyatomic ion. In general, every chemicals which is called 'Borate' is known that each of them has different molecular structure of the anion. Borates are chemical compounds which contains boron with boron an oxidation state +3. Borates are generally used by combining with sodium borates (borax) and boron compounds. Borates, sodium borates (borax) and boron compounds are generally used for laundries, soak, and diapers. They also used as tan ingredient in cosmetics, medicines, ceramics and building materials.
Properties
Chemical
Borate is an ion, which means it can't be exist itself. It has to be combined with other chemicals to exist. In general, every chemicals which is called 'Borate' are known that each of them have different molecular structure of the anion. Borates are chemical compounds which contains boron oxyanions with boron in oxidation state +3. [1] The molecular formula of Borate is BO3, It's average mass is 58.811 Da and monoisotopic mass is 58.995697 Da. Alkali metal salts are colorless, generally having water of crystallization, and are water-soluble. Solution indicates alkaline because of hydrolysis. Other metal salts are generally poorly soluble. [2]
Physical
The general form for sodium borates (borax) is vitreous, resinous, and earthy. Their physical properties are translucent, opaque. They are colorless, grey, white, yellowish, seldom bluish or greenish; colorless in transmitted light, and they include white lines. The hardness of sodium borate is 2 - 2½, and its tenacity is brittle. Its fracture is conchoidal, and density is 1.715(2) g/cm3 (measured), 1.7 g/cm3 (calculated). Density is measured with the pure chemical compound [3]
Natural Occurrences
There are over one hundred borate minerals in the world, including boracite, borax, colemanite, inyoite, kernite, ludwigite, priceite, sussexite, tincalconite, and ulexite. These all include borate anion. Boracite is colorless or white, and it is isolated and embedded. Borax is also colorless or seldomly grayish or greenish, and its form is crystal which is shortly prismatic. Colemanite is also colorless white, and its form is same as borax, but massive. Inyoite is also colorless but it becomes white and cloudy after partial dehydration. The form of it is short prisms and coarse crystal aggregates. Kernite is also colorless, and has very large crystals. And it's fibrous and cleavable. Ludwigite is dark green to coal black, and is fibrous masses. It is rosettes. Priceite is white, soft and chalky to hard and tough nodules. Sussexite is white to straw-yellow,and fibrous mineral. Tincalconite is white in natural, but colorless when it formed artificially. It is found in nature as a fine-grained powder. Ulexite is colorless or white, and is small nodular, rounded, or lens-like crystal aggregates. There are two structures in borate minerals, BO3 triangle and BO4 tetrahedron. Oxygen or hydroxyl groups are respectively located on the triangle's vertices or tetrahedron's corner with boron centered in it. Vertices are able to share an oxygen atom to form extended boron oxygen network. The size of boron oxygen complexity in any one mineral generally decreases. It happens with increasing temperature, and pressure which the mineral forms at. [4]
Sodium borate (borax) is naturally made from the evaporation of seasonal lakes. They are usually found in Turkey, California, Southwestern United States, the Atacama desert in Chile, and in Tibet and Romania. Sodium borate is basically a salt that made from lake evaporation. However, it would be possible that top of the scale of sodium borate would be something like mercury, which has ability to wreaks havoc on human brain functions. Therefore it's impossible for human to eat it. [5] Borates are generally used by combining with sodium borates (borax) and boron compounds
Uses
A long time ago, sodium borates were considered as a magical crystal which was used to help digestion, keep milk sweet and also cure epilepsy(falling sickness). [6] Sodium borate (borax), which is one of the most usefully and generally used kind of borates in our life, has lots of uses and effectiveness. And also, they are one of the most useful and generally used kind of borates in our life. Sodium borates are possibly effective for vaginal infections.
Borates, sodium borates (borax) and boron compounds are generally used for the laundries, soak, and diapers. They are also used as the ingredient in cosmetics, medicines, ceramics and building materials. Borates are also used for making glasses, especially fiberglass and gorilla glass.
Acid from borate can successfully treat candidiasis(yeast infections). They are also possibly ineffective for athletic performance. People who have boron deficiency put boron into their mouth to prevent it, but this would disturb their athletic performances. Taking boron with mouth would not improve their body mass, muscular mass, and testosterone levels in male bodybuilders. Sodium borates also have insufficient evidence for osteoarthritis, and osteoporosis. [7]
Side effects of Borate
Borates are generally used around us. (Antiseptic...etc) However, it can badly affect people like examples below.
-Skin problems
-Reproductive dysfunction
-Eye diseases
-harm soils [8]
Video
One of the good effects of borate (Benefit of adding borate in the pool)
References
- ↑ Borate Princeton. Web. last accessed on 12 January 2015. Author Unknown.
- ↑ 네이년 화학대사전. Web. last accessed on 12 January 2015. Author Unknown.
- ↑ Borax Physical Properties of Borax. Web. last accessed on 27 January 2015. Author Unknown
- ↑ borate mineral encyclopaedia britannica. Web. last accessed on 29, January, 2015.The Editors of Encyclopædia Britannica.
- ↑ Borax What_is_borax. Web. last accessed on 27 January 2015. Author Unknown
- ↑ Boron Find_a_Vitamin_or_Supplement. Web. last accessed on 13 January 2015. Author Unknown
- ↑ Getting_to_the_Bottom_of_Borax Is it Safe or Not?. Web. uploaded on 3, August, 2011. Crunch Betty
| ||||||||||||||

