Uploads by Chemicalinterest
From CreationWiki
Jump to navigationJump to search
This special page shows all uploaded files.
| Date | Name | Thumbnail | Size | Description | Versions |
|---|---|---|---|---|---|
| 15:54, 24 February 2011 | Aluminium acetate.JPG (file) |  |
138 KB | Aluminium acetate category:aluminium category:acetate | 1 |
| 20:46, 19 February 2011 | Disodium phosphate.JPG (file) |  |
130 KB | Disodium phosphate category:sodium category:phosphate | 1 |
| 19:17, 16 February 2011 | Tin(II) sulfate.JPG (file) |  |
82 KB | Tin(II) sulfate made by reacting tin with copper(II) sulfate. category:tin category:sulfate | 1 |
| 15:29, 16 February 2011 | Ammonium chloride.JPG (file) |  |
99 KB | Ammonium chloride category:ammonium category:chloride | 1 |
| 15:28, 16 February 2011 | Ammonium carbonate.JPG (file) |  |
151 KB | Ammonium carbonate category:ammonium category:carbonate | 1 |
| 13:58, 16 February 2011 | Calcium acetate.JPG (file) |  |
252 KB | Calcium acetate made by dissolving calcium carbonate in acetic acid and crystallizing | 2 |
| 01:19, 15 February 2011 | Cobalt(II) borate.JPG (file) |  |
46 KB | Cobalt(II) borate category:cobalt category:borate | 1 |
| 01:19, 15 February 2011 | Cobalt chloride (2).JPG (file) |  |
106 KB | Cobalt chloride category:cobalt category:chloride | 1 |
| 00:01, 15 February 2011 | Cobalt(II) acetate (2).JPG (file) |  |
244 KB | Cobalt(II) acetate category:cobalt | 1 |
| 19:16, 14 February 2011 | Potassium chloride (2).JPG (file) |  |
70 KB | Potassium chloride category:potassium category:chloride | 1 |
| 19:15, 14 February 2011 | Sodium hypochlorite solution.JPG (file) |  |
92 KB | A solution of sodium hypochlorite (household bleach) | 1 |
| 19:15, 14 February 2011 | Cobalt(II) hydroxide (2).JPG (file) |  |
229 KB | Cobalt(II) hydroxide category:cobalt | 1 |
| 19:14, 14 February 2011 | Cobalt(II) acetate.JPG (file) | 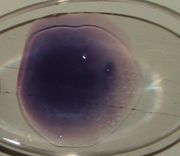 |
75 KB | Cobalt(II) acetate dried on a lens. category:cobalt | 1 |
| 19:13, 14 February 2011 | Lithium acetate.JPG (file) |  |
62 KB | Lithium acetate category:lithium | 1 |
| 19:12, 14 February 2011 | Ammonium acetate.JPG (file) |  |
146 KB | Ammonium acetate category:ammonium | 1 |
| 19:12, 14 February 2011 | Sodium acetate (2).JPG (file) |  |
86 KB | Sodium acetate category:sodium | 1 |
| 19:11, 14 February 2011 | Purified tin metal 2.JPG (file) |  |
52 KB | Tin metal produced by electrolysis category:tin | 1 |
| 19:11, 14 February 2011 | Lithium chloride 2.jpg (file) |  |
19 KB | Lithium chloride category:lithium category:chloride | 1 |
| 19:09, 14 February 2011 | Sodium ascorbate.JPG (file) |  |
11 KB | An oxidized caramel textured form of sodium ascorbate category:sodium | 1 |
| 12:30, 11 February 2011 | Copper sulfate.JPG (file) |  |
167 KB | Copper sulfate crystals category:sulfate category:copper | 1 |
| 12:29, 11 February 2011 | Copper(II) sulfate.JPG (file) |  |
73 KB | Copper(II) sulfate powder category:copper category:sulfate | 1 |
| 12:01, 7 February 2011 | Calcium hydroxide 2.JPG (file) |  |
200 KB | Calcium hydroxide powder from a chemistry set | 1 |
| 11:59, 7 February 2011 | Calcium oxide.JPG (file) |  |
132 KB | Calcium oxide, about 20 years old, from a chemistry set | 1 |
| 11:56, 7 February 2011 | Sodium sulfate.JPG (file) |  |
65 KB | Sodium sulfate made by drying the filtrate produced when copper sulfate and sodium bicarbonate react. Copper bicarbonate was also produced in this reaction which readily decomposed to copper carbonate, with the production of carbon dioxide gas. [[categor | 1 |
| 01:37, 4 February 2011 | Aluminium sulfate.JPG (file) |  |
118 KB | Aluminium sulfate | 1 |
| 01:36, 4 February 2011 | Bismuth stain.JPG (file) |  |
170 KB | Stain made by bismuth on paper category:bismuth | 1 |
| 01:35, 4 February 2011 | Nickel(II) oxide.JPG (file) |  |
76 KB | Nickel(II) oxide category:nickel category:oxide | 1 |
| 23:52, 2 February 2011 | Iron(III) oxide (2).JPG (file) |  |
385 KB | Iron(III) oxide powder | 1 |
| 23:51, 2 February 2011 | Lithium chunk (3).JPG (file) |  |
110 KB | A chunk of lithium covered with a superficial oxide and nitride layer. | 1 |
| 16:37, 29 January 2011 | Copper(II) chloride crystals.JPG (file) |  |
610 KB | Copper(II) chloride crystals on a watch glass category:copper category:chloride | 1 |
| 12:43, 26 January 2011 | Baking powder crumbs.JPG (file) |  |
875 KB | This is made when baking powder is reacted with water; a mixture of dicalcium phosphate, disodium phosphate, and cornstarch. category:phosphate | 1 |
| 01:36, 26 January 2011 | Iron(III) phosphate.JPG (file) |  |
94 KB | Iron(III) phosphate. This is used for iron nutrition in some foods. category:iron category:phosphate | 1 |
| 01:35, 26 January 2011 | Silver(I) oxide 2.JPG (file) |  |
92 KB | Silver(I) oxide made by reacting concentrated lithium hydroxide with silver nitrate. There was a microscopic amount of silver nitrate left so the amount of silver(I) oxide is very small. | 1 |
| 01:34, 26 January 2011 | Copper(II) oxide (2).JPG (file) |  |
61 KB | Copper(II) oxide powder. This was made by electrolyzing copper in sodium carbonate and heating the precipitate formed. category:copper category:oxide | 1 |
| 01:33, 26 January 2011 | Metals and their hydroxides.JPG (file) |  |
1,010 KB | An assortment of metals and their hydroxides produced by placing them on the anode of a sodium-carbonate-electrolyte electrolysis system in lids. Aluminium does not form its hydroxide well this way. With a longer wait all of the others could have formed m | 1 |
| 01:31, 26 January 2011 | Zinc phosphate.JPG (file) |  |
219 KB | Zinc phosphate category:zinc category:phosphate | 1 |
| 16:00, 25 January 2011 | Ascorbic acid.JPG (file) |  |
331 KB | Ascorbic acid from vitamin supplement | 1 |
| 15:59, 25 January 2011 | Copper(II) borate.JPG (file) |  |
69 KB | Copper(II) borate made by reaction of copper(II) chloride with borax. | 1 |
| 15:58, 25 January 2011 | Copper(II) silicate.JPG (file) |  |
113 KB | Copper(II) silicate made by reacting copper(II) chloride with sodium silicate category:silicate category:copper | 1 |
| 15:57, 25 January 2011 | Aluminium chloride.JPG (file) |  |
110 KB | Saturated solution of aluminium chloride with colorless aluminium chloride crystals. The solution is yellow because of impurities in the aluminium foil used. This is very common. The aluminium chloride was made by reacting aluminium with copper(II) chlori | 1 |
| 15:56, 25 January 2011 | Magnesium carbonate.JPG (file) |  |
81 KB | Magnesium carbonate was generated by reacting magnesium sulfate with sodium carbonate. category:magnesium category:carbonate | 1 |
| 15:55, 25 January 2011 | Zinc carbonate.JPG (file) |  |
132 KB | Zinc carbonate generated by reacting zinc with hydrochloric acid to make zinc chloride. The zinc chloride is reacted with sodium carbonate to make zinc carbonate. | 1 |
| 15:54, 25 January 2011 | Cuprous oxide microcrystalline 3.JPG (file) |  |
92 KB | A puddle of copper(I) oxide in its yellow form. This was made by reacting concentrated lithium hydroxide with copper(I) chloride solution. category:oxide category:copper | 1 |
| 12:39, 23 January 2011 | Tin dioxide 2.JPG (file) |  |
162 KB | A mixture of tin dioxide and tin(IV) carbonate made by reacting a solution of tin(IV) chloride and hydrochloric acid/hydrogen peroxide with sodium carbonate. The tin(IV) chloride was made by dissolving tin in a hydrochloric acid/hydrogen peroxide solution | 1 |
| 12:38, 23 January 2011 | Tin dioxide (2).JPG (file) |  |
22 KB | Pure tin dioxide. This was made by dissolving tin in a mixture of hydrogen peroxide and hydrochloric acid. This mixture was reacted with ammonia to make tin dioxide, which was filtered and dried. category:tin category:oxide | 1 |
| 13:33, 22 January 2011 | Cuprous oxide.JPG (file) | 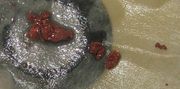 |
197 KB | Chunks of copper(I) oxide formed by the reaction of aluminium metal with concentrated copper(II) chloride solution. Hydrogen gas and aluminium(III) chloride are also produced. | 1 |
| 21:44, 21 January 2011 | Cobalt hydroxides.JPG (file) |  |
913 KB | Two forms of cobalt(II) hydroxide category:cobalt | 1 |
| 18:19, 21 January 2011 | Antimony powder 2.JPG (file) |  |
245 KB | Antimony powder. This is made by dissolving pewter in hydrochloric acid. The tin dissolves, leaving the antimony behind. The copper may dissolve, but it is reduced by the tin(II) ions to copper(I), which is colorless and soluble in hydrochloric acid. [[c | 1 |
| 18:07, 21 January 2011 | Aluminium hydroxide (2).JPG (file) |  |
21 KB | Aluminium hydroxide. This was made by dissolving aluminium in dilute hydrochloric acid (not concentrated) and reacting the aluminium chloride with ammonium hydroxide. The product is a gel that dries to a powder. category:aluminium [[category:hydroxid | 1 |
| 18:06, 21 January 2011 | Lead(II) hydroxide.JPG (file) |  |
116 KB | A mixture of lead(II) chloride, lead(II) carbonate, and lead(II) hydroxide. Lead(II) hydroxide only forms at very high pH's. | 1 |